Transient elevation of glycolysis confers radio-resistance by facilitating DNA repair in cells
- PMID: 25925410
- PMCID: PMC4425929
- DOI: 10.1186/s12885-015-1368-9
Transient elevation of glycolysis confers radio-resistance by facilitating DNA repair in cells
Abstract
Background: Cancer cells exhibit increased glycolysis for ATP production (the Warburg effect) and macromolecular biosynthesis; it is also linked with therapeutic resistance that is generally associated with compromised respiratory metabolism. Molecular mechanisms underlying radio-resistance linked to elevated glycolysis remain incompletely understood.
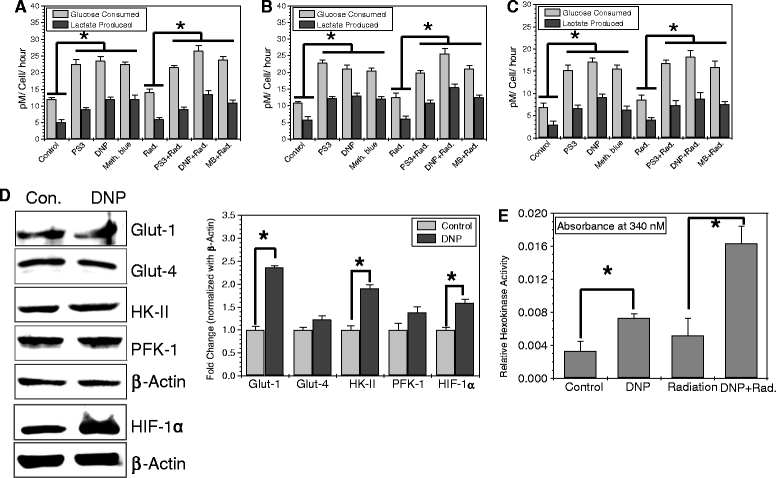
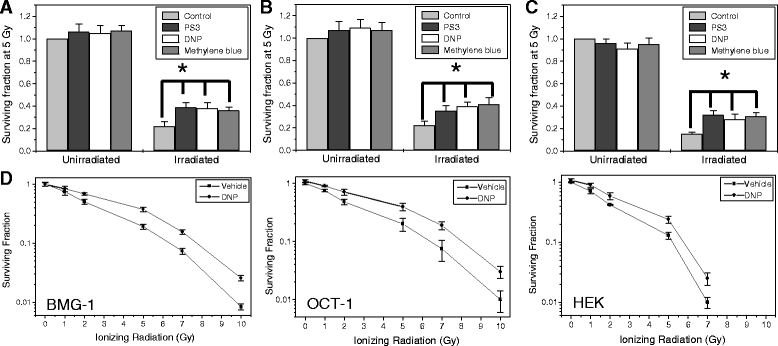
Methods: We stimulated glycolysis using mitochondrial respiratory modifiers (MRMs viz. di-nitro phenol, DNP; Photosan-3, PS3; Methylene blue, MB) in established human cell lines (HEK293, BMG-1 and OCT-1). Glucose utilization and lactate production, levels of glucose transporters and glycolytic enzymes were investigated as indices of glycolysis. Clonogenic survival, DNA repair and cytogenetic damage were studied as parameters of radiation response.
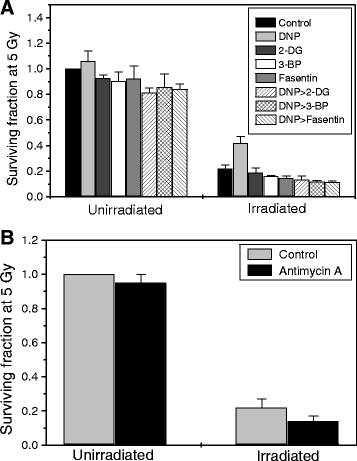
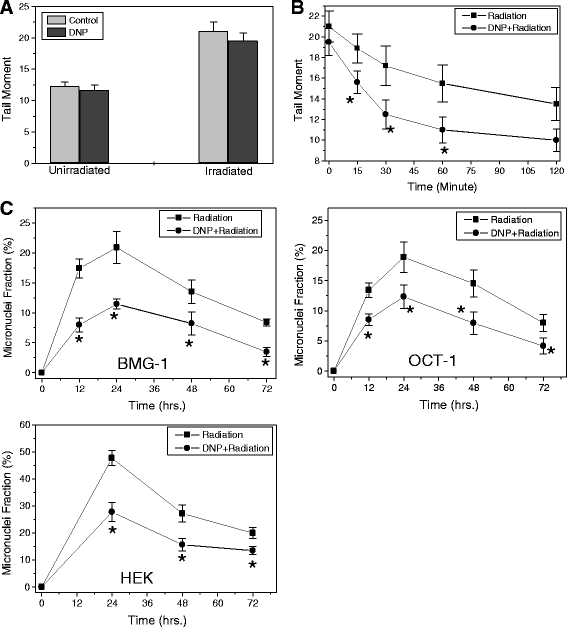
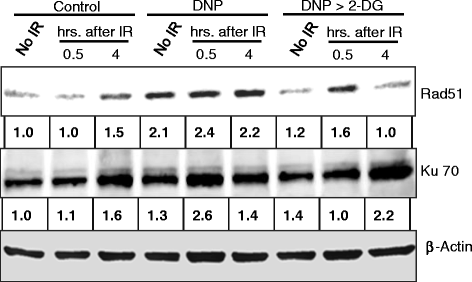
Results: MRMs induced the glycolysis by enhancing the levels of two important regulators of glucose metabolism GLUT-1 and HK-II and resulted in 2 fold increase in glucose consumption and lactate production. This increase in glycolysis resulted in resistance against radiation-induced cell death (clonogenic survival) in different cell lines at an absorbed dose of 5 Gy. Inhibition of glucose uptake and glycolysis (using fasentin, 2-deoxy-D-glucose and 3-bromopyruvate) in DNP treated cells failed to increase the clonogenic survival of irradiated cells, suggesting that radio-resistance linked to inhibition of mitochondrial respiration is glycolysis dependent. Elevated glycolysis also facilitated rejoining of radiation-induced DNA strand breaks by activating both non-homologous end joining (NHEJ) and homologous recombination (HR) pathways of DNA double strand break repair leading to a reduction in radiation-induced cytogenetic damage (micronuclei formation) in these cells.
Conclusions: These findings suggest that enhanced glycolysis generally observed in cancer cells may be responsible for the radio-resistance, partly by enhancing the repair of DNA damage.
Figures

References
-
- Schwaab J, Horisberger K, Ströbel P, Bohn B, Gencer D, Kähler G, et al. Erben Expression of Transketolase like gene 1 (TKTL1) predicts disease-free survival in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy. BMC Cancer. 2011;11:363. doi: 10.1186/1471-2407-11-363. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

