US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines
- PMID: 25925419
- PMCID: PMC4838063
- DOI: 10.1093/jnci/djv086
US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines
Abstract
Background: This study sought to determine the prevaccine type-specific prevalence of human papillomavirus (HPV)-associated cancers in the United States to evaluate the potential impact of the HPV types in the current and newly approved 9-valent HPV vaccines.
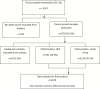
Methods: The Centers for Disease Control and Prevention partnered with seven US population-based cancer registries to obtain archival tissue for cancers diagnosed from 1993 to 2005. HPV testing was performed on 2670 case patients that were fairly representative of all participating cancer registry cases by age and sex. Demographic and clinical data were evaluated by anatomic site and HPV status. Current US cancer registry data and the detection of HPV types were used to estimate the number of cancers potentially preventable through vaccination.
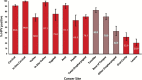
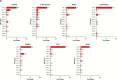
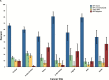
Results: HPV DNA was detected in 90.6% of cervical, 91.1% of anal, 75.0% of vaginal, 70.1% of oropharyngeal, 68.8% of vulvar, 63.3% of penile, 32.0% of oral cavity, and 20.9% of laryngeal cancers, as well as in 98.8% of cervical cancer in situ (CCIS). A vaccine targeting HPV 16/18 potentially prevents the majority of invasive cervical (66.2%), anal (79.4%), oropharyngeal (60.2%), and vaginal (55.1%) cancers, as well as many penile (47.9%), vulvar (48.6%) cancers: 24 858 cases annually. The 9-valent vaccine also targeting HPV 31/33/45/52/58 may prevent an additional 4.2% to 18.3% of cancers: 3944 cases annually. For most cancers, younger age at diagnosis was associated with higher HPV 16/18 prevalence. With the exception of oropharyngeal cancers and CCIS, HPV 16/18 prevalence was similar across racial/ethnic groups.
Conclusions: In the United States, current vaccines will reduce most HPV-associated cancers; a smaller additional reduction would be contributed by the new 9-valent vaccine.
Published by Oxford University Press 2015. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures


Comment in
-
The beginning of the end: vaccine prevention of HPV-driven cancers.J Natl Cancer Inst. 2015 Apr 24;107(6):djv128. doi: 10.1093/jnci/djv128. J Natl Cancer Inst. 2015. PMID: 25911509 No abstract available.
References
-
- Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. - PubMed
-
- Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–F23. - PubMed
-
- International Agency for Research on Cancer IARC. Monographs on the evaluation of carcinogenic risks to humans: biologic agents. Human papillomaviruses. Vol. 100B. Lyon, France: IARC; 2012. http://monographs.iarc.fr/ENG/Monographs/vol100B/mono100B-11.pdf
-
- Bouvard V, Baan R, Straif K, et al. A review of human carcinogens-Part B: biological agents. The Lancet Oncol. 2009; 10(4):321–322. - PubMed
-
- Doorbar J, Quint W, Banks L, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–F70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

