A circuit mechanism for differentiating positive and negative associations
- PMID: 25925480
- PMCID: PMC4418228
- DOI: 10.1038/nature14366
A circuit mechanism for differentiating positive and negative associations
Abstract
The ability to differentiate stimuli predicting positive or negative outcomes is critical for survival, and perturbations of emotional processing underlie many psychiatric disease states. Synaptic plasticity in the basolateral amygdala complex (BLA) mediates the acquisition of associative memories, both positive and negative. Different populations of BLA neurons may encode fearful or rewarding associations, but the identifying features of these populations and the synaptic mechanisms of differentiating positive and negative emotional valence have remained unknown. Here we show that BLA neurons projecting to the nucleus accumbens (NAc projectors) or the centromedial amygdala (CeM projectors) undergo opposing synaptic changes following fear or reward conditioning. We find that photostimulation of NAc projectors supports positive reinforcement while photostimulation of CeM projectors mediates negative reinforcement. Photoinhibition of CeM projectors impairs fear conditioning and enhances reward conditioning. We characterize these functionally distinct neuronal populations by comparing their electrophysiological, morphological and genetic features. Overall, we provide a mechanistic explanation for the representation of positive and negative associations within the amygdala.
Figures



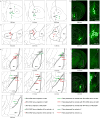
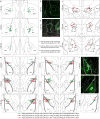

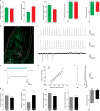

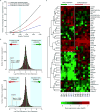




Comment in
-
Neural circuits: Pain or pleasure?Nat Rev Neurosci. 2015 Jun;16(6):316. doi: 10.1038/nrn3975. Nat Rev Neurosci. 2015. PMID: 25991440 No abstract available.
References
-
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. - PubMed
-
- Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. - PubMed
-
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic Receptor Trafficking Underlying a Form of Associative Learning. Science. 2005;308:83–88. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

