Comprehensive molecular characterization of human adipocytes reveals a transient brown phenotype
- PMID: 25925588
- PMCID: PMC4438513
- DOI: 10.1186/s12967-015-0480-6
Comprehensive molecular characterization of human adipocytes reveals a transient brown phenotype
Abstract
Background: Functional brown adipose tissue (BAT), involved in energy expenditure, has recently been detected in substantial amounts in adults. Formerly overlooked BAT has now become an attractive anti-obesity target.
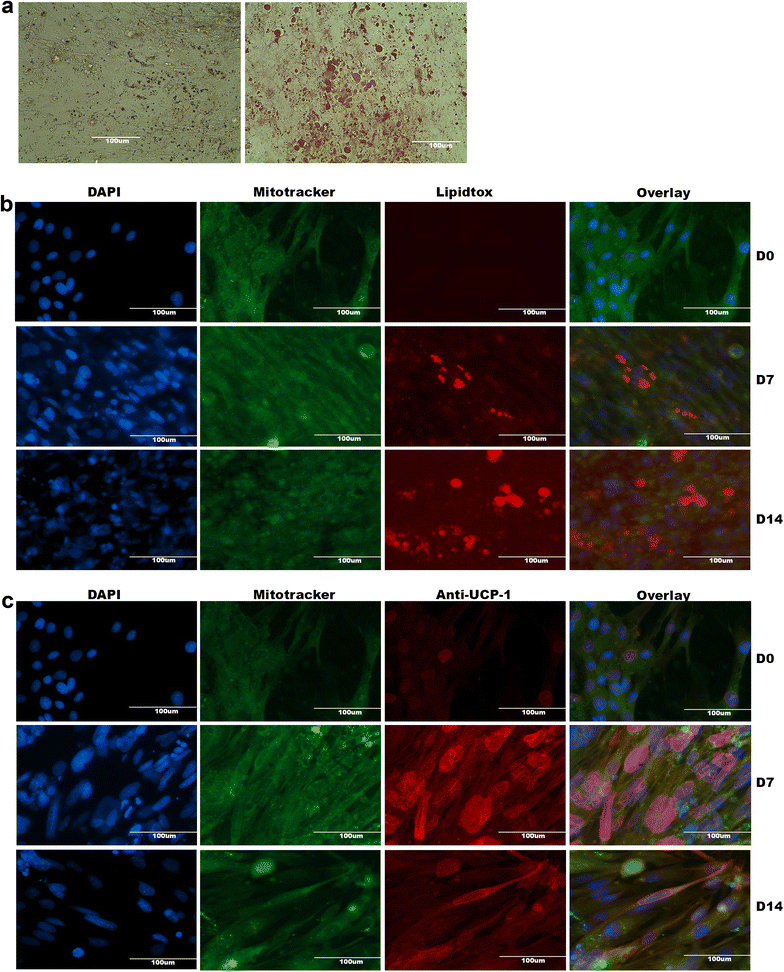
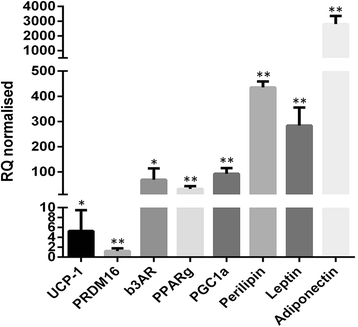
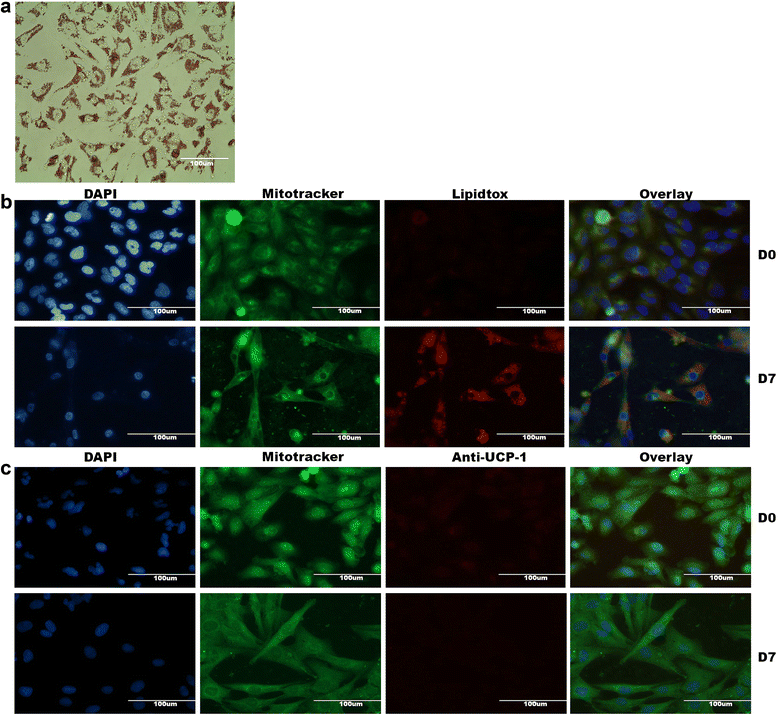
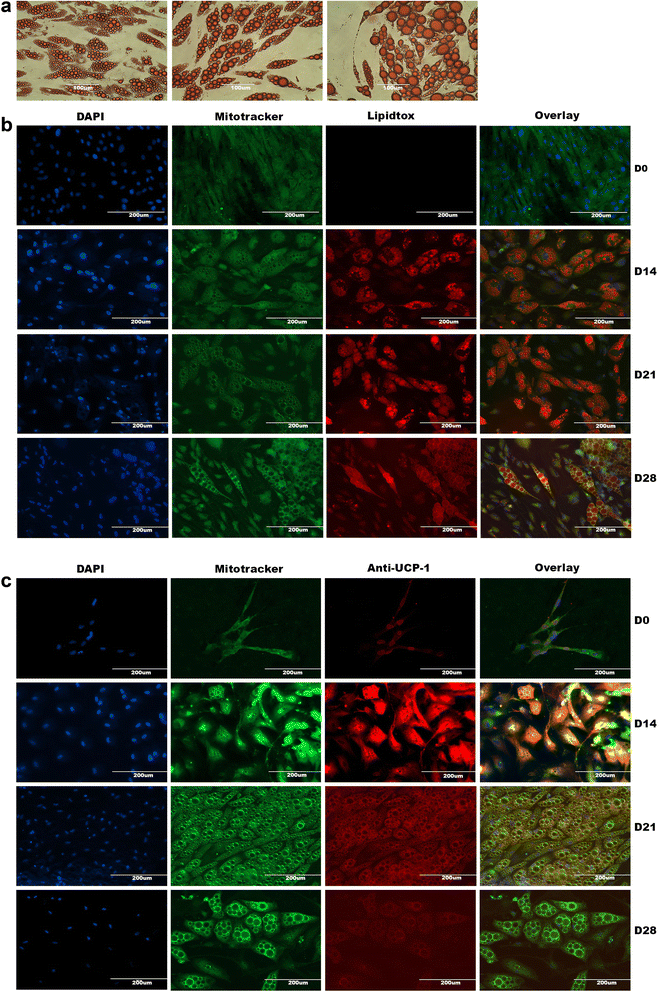
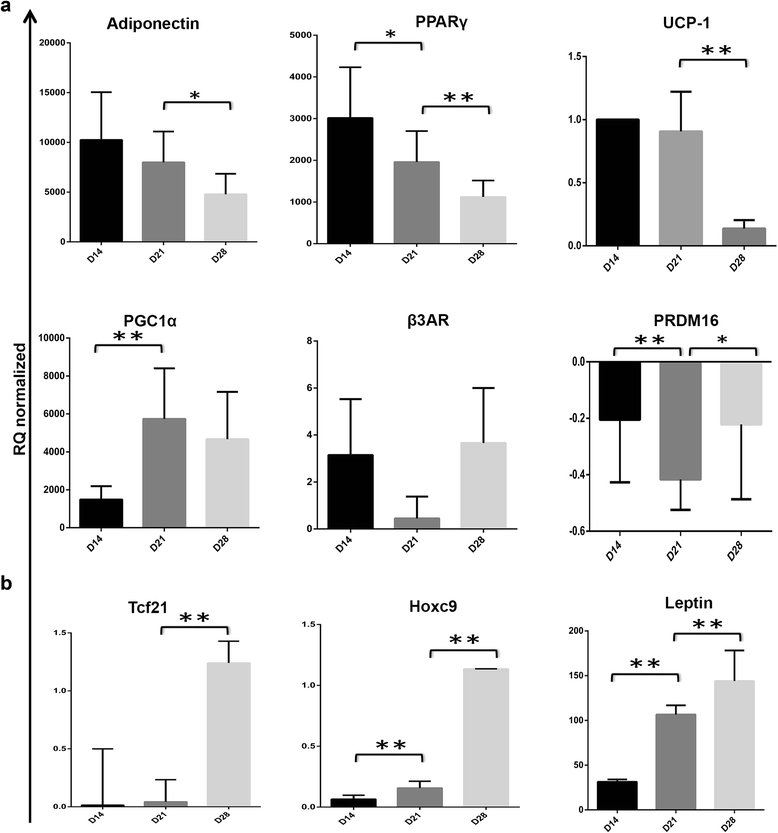
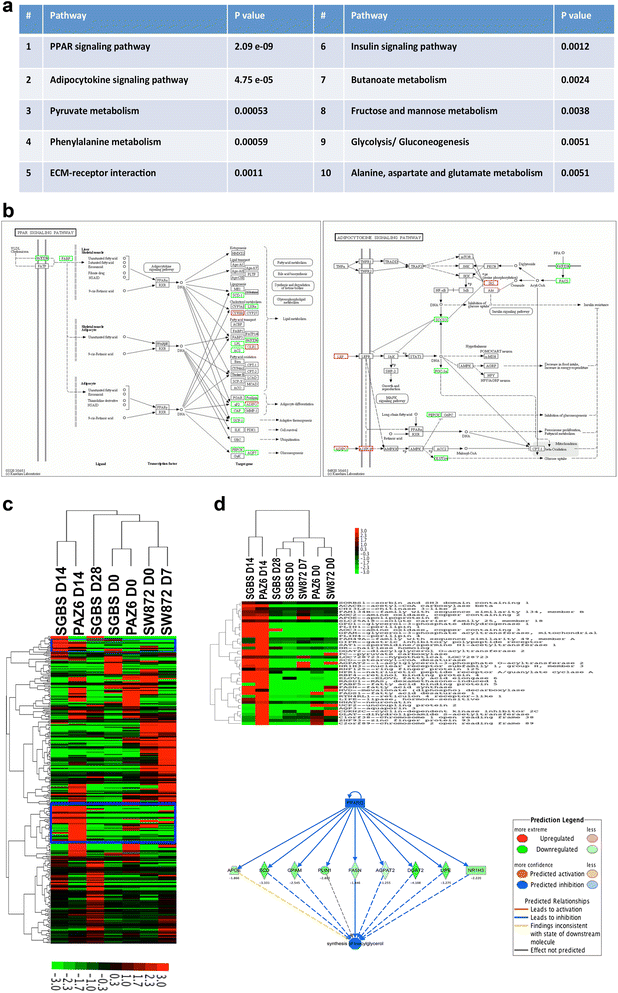
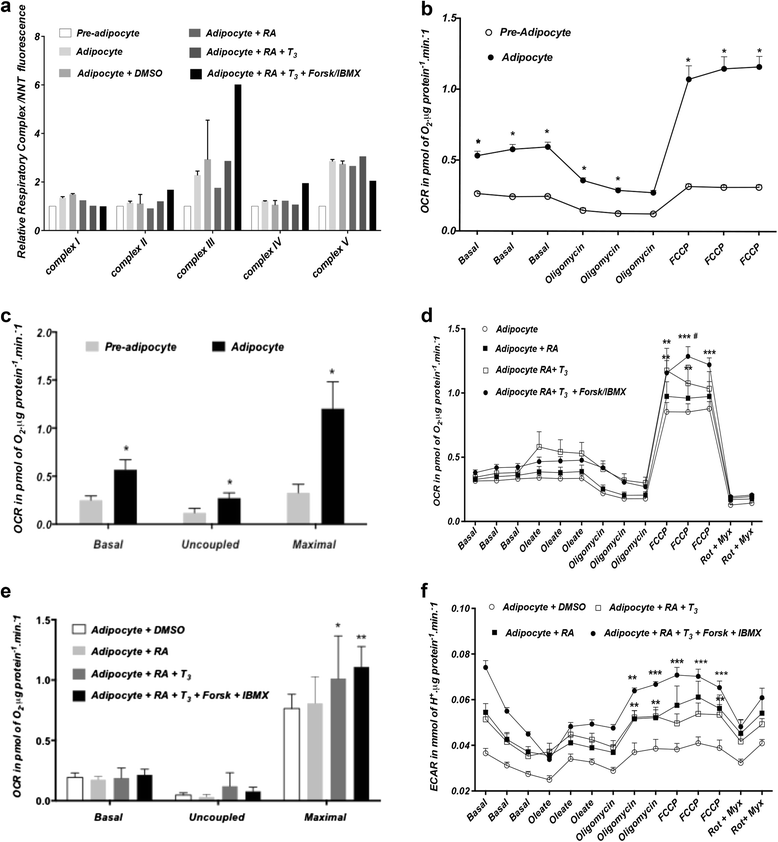
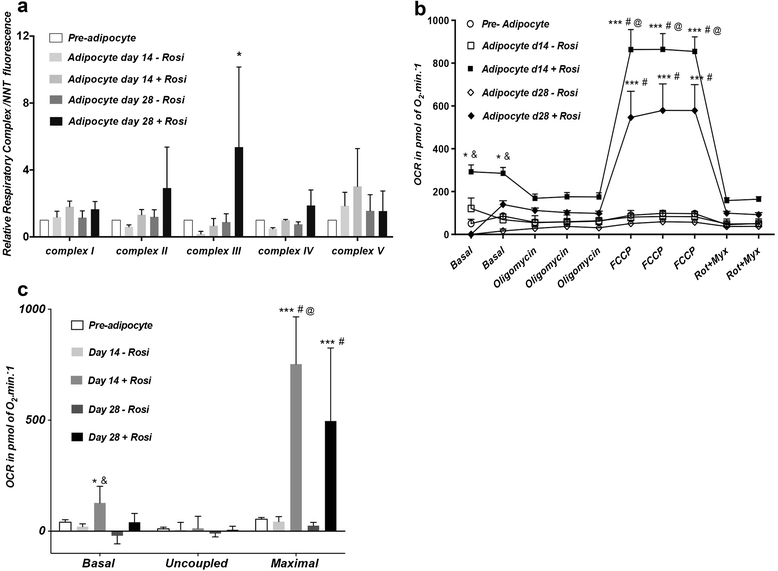
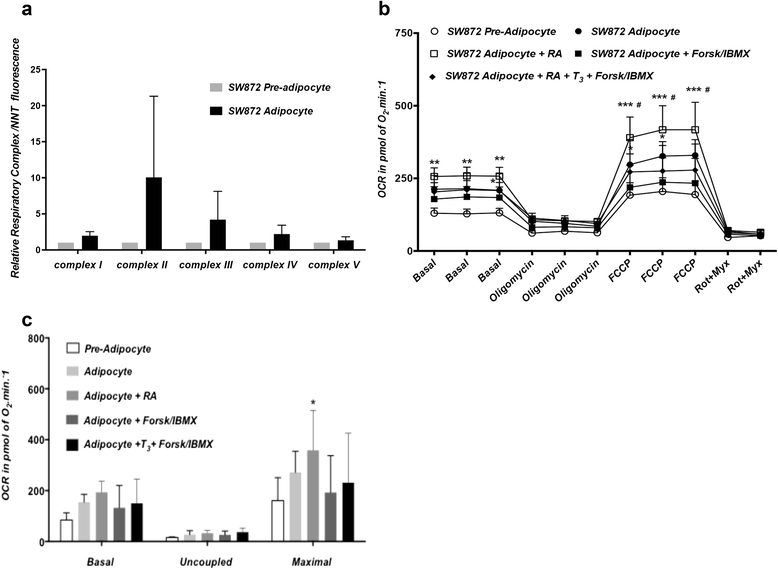
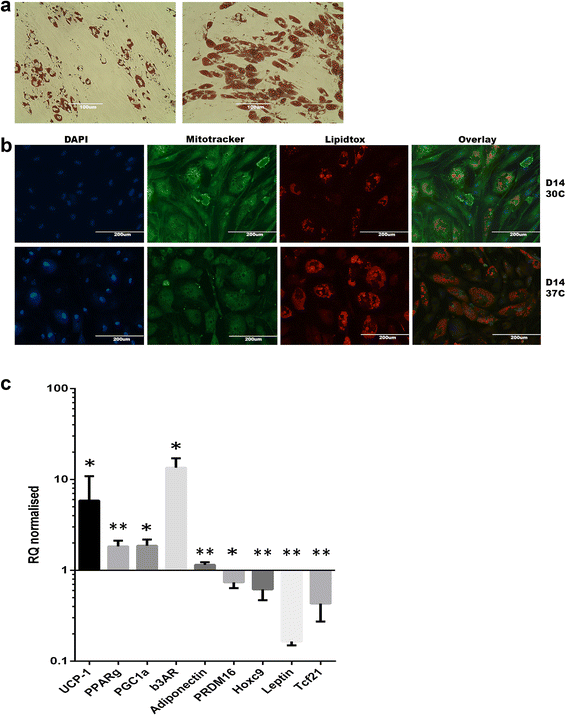
Methods and results: Molecular characterization of human brown and white adipocytes, using a myriad of techniques including high-throughput RNA sequencing and functional assays, showed that PAZ6 and SW872 cells exhibit classical molecular and phenotypic markers of brown and white adipocytes, respectively. However, the pre-adipocyte cell line SGBS presents a versatile phenotype. A transit expression of classical brown markers such as UCP1 and PPARγ peaked and declined at day 28 post-differentiation initiation. Conversely, white adipocyte markers, including Tcf21, showed reciprocal behavior. Interestingly, leptin levels peaked at day 28 whereas the highest adiponectin mRNA levels were detected at day 14 of differentiation. Phenotypic analysis of the abundance and shape of lipid droplets were consistent with the molecular patterns. Accordingly, the oxidative capacity of SGBS adipocytes peaked on differentiation day 14 and declined progressively towards differentiation day 28.
Conclusions: Our studies have unveiled a new phenotype of human adipocytes, providing a tool to identify molecular gene expression patterns and pathways involved in the conversion between white and brown adipocytes.
Figures

References
-
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

