Statin-induced anti-proliferative effects via cyclin D1 and p27 in a window-of-opportunity breast cancer trial
- PMID: 25925673
- PMCID: PMC4424530
- DOI: 10.1186/s12967-015-0486-0
Statin-induced anti-proliferative effects via cyclin D1 and p27 in a window-of-opportunity breast cancer trial
Abstract
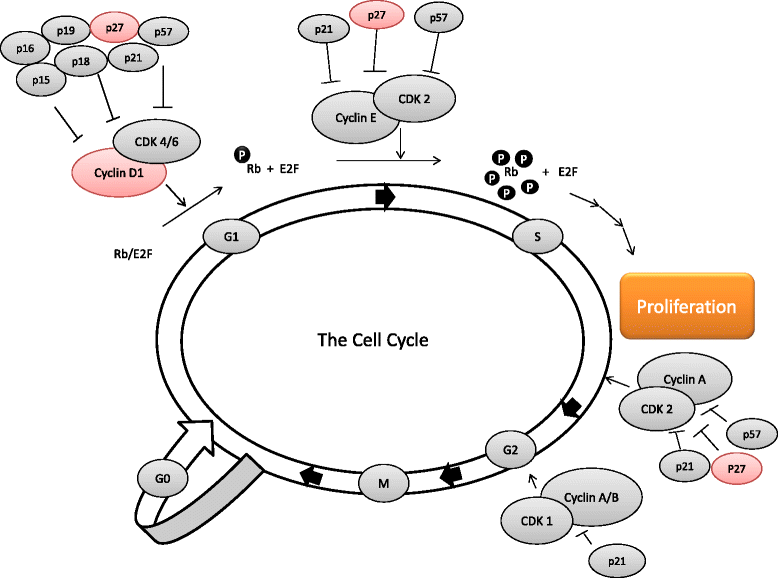
Purpose: Cholesterol lowering statins have been demonstrated to exert anti-tumoral effects on breast cancer by decreasing proliferation as measured by Ki67. The biological mechanisms behind the anti-proliferative effects remain elusive. The aim of this study was to investigate potential statin-induced effects on the central cell cycle regulators cyclin D1 and p27.
Experimental design: This phase II window-of-opportunity trial (Trial registration: ClinicalTrials.gov NCT00816244 , NIH) included 50 patients with primary invasive breast cancer. High-dose atorvastatin (80 mg/day) was prescribed to patients for two weeks prior to surgery. Paired paraffin embedded pre- and post-statin treatment tumor samples were analyzed using immunohistochemistry for the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and the cell cycle regulators cyclin D1 and p27. Corresponding frozen tumor sample pairs were analyzed for expression of the genes coding for cyclin D1 and p27, CCND1 and CDKN1B, respectively.
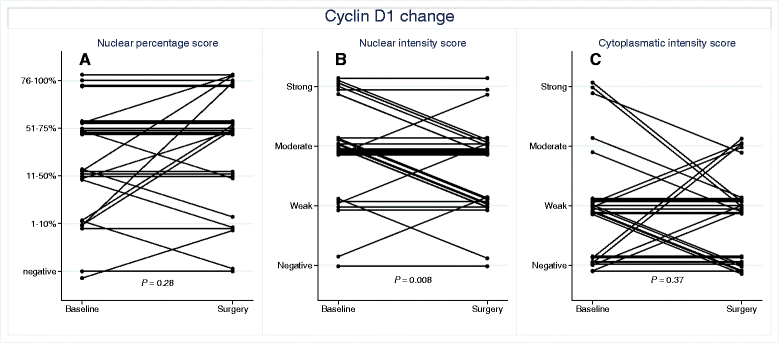
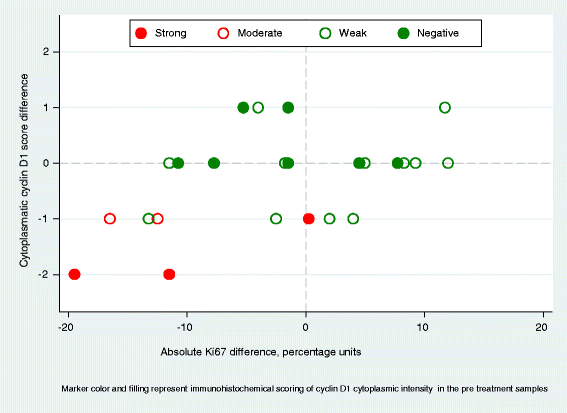
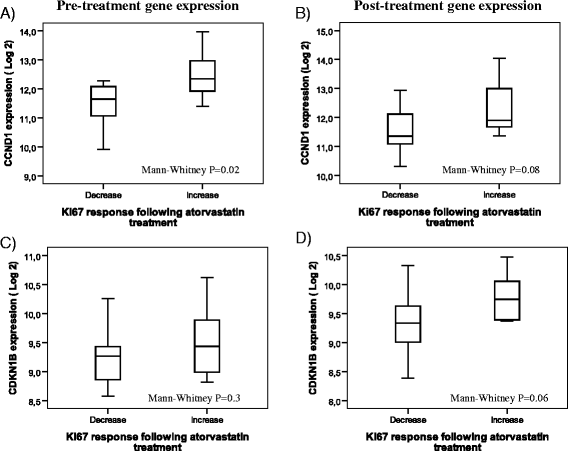
Results: Forty-two patients completed all study parts, and immunohistochemical evaluation of ER and PR was achievable in 30 tumor pairs, HER2 in 29 tumor pairs, cyclin D1 in 30 tumor pairs and p27 in 33 tumor pairs. The expression of ER, PR and HER2 did not change significantly following atorvastatin treatment. Cyclin D1 expression in terms of nuclear intensity was significantly decreased (P = 0.008) after statin treatment in paired tumor samples. The protein expression of the tumor suppressor p27, evaluated either as the fraction of stained tumor cells or as cytoplasmic intensity, increased significantly (P = 0.03 and P = 0.02, respectively). At the transcriptional level, no significant differences in mRNA expression were detected for cyclin D1 (CCND1) and p27 (CDKN1B). However, CCND1 expression was lower in tumors responding to atorvastatin treatment with a decrease in proliferation although not significantly (P = 0.08).
Conclusions: We have previously reported statin-induced anti-proliferative effects in breast cancer. This study suggests that cell cycle regulatory effects may contribute to these anti-proliferative effects via cyclin D1 and p27.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

