Adverse Outcomes of Tacrolimus Withdrawal in Immune-Quiescent Kidney Transplant Recipients
- PMID: 25925687
- PMCID: PMC4657844
- DOI: 10.1681/ASN.2014121234
Adverse Outcomes of Tacrolimus Withdrawal in Immune-Quiescent Kidney Transplant Recipients
Abstract
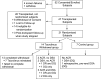
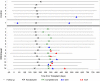
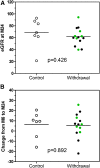
Concerns about adverse effects of calcineurin inhibitors (CNIs) have prompted development of protocols that minimize their use. Whereas previous CNI withdrawal trials in heterogeneous cohorts showed unacceptable rates of acute rejection (AR), we hypothesized that we could identify individuals capable of tolerating CNI withdrawal by targeting immunologically quiescent kidney transplant recipients. The Clinical Trials in Organ Transplantation-09 Trial was a randomized, prospective study of nonsensitized primary recipients of living donor kidney transplants. Subjects received rabbit antithymocyte globulin, tacrolimus, mycophenolate mofetil, and prednisone. Six months post-transplantation, subjects without de novo donor-specific antibodies (DSAs), AR, or inflammation at protocol biopsy were randomized to wean off or remain on tacrolimus. The intended primary end point was the change in interstitial fibrosis/tubular atrophy score between implantation and 24-month protocol biopsies. Serially collected urine CXCL9 ELISA results were correlated with outcomes. The study was terminated prematurely because of unacceptable rates of AR (4 of 14) and/or de novo DSAs (5 of 14) in the tacrolimus withdrawal arm. Positive urinary CXCL9 predated clinical detection of AR by a median of 15 days. Analyses showed that >16 HLA-DQ epitope mismatches and pretransplant, peripheral blood, donor-reactive IFN-γ ELISPOT assay results correlated with development of DSAs and/or AR on tacrolimus withdrawal. Although data indicate that urinary CXCL9 monitoring, epitope mismatches, and ELISPOT assays are potentially informative, complete CNI withdrawal must be strongly discouraged in kidney transplant recipients who are receiving standard-of-care immunosuppression, including those who are deemed to be immunologically quiescent on the basis of current clinical and laboratory criteria.
Keywords: immunosuppression; rejection; renal transplantation.
Copyright © 2015 by the American Society of Nephrology.
Figures


Comment in
-
Moving Beyond Minimization Trials in Kidney Transplantation.J Am Soc Nephrol. 2015 Dec;26(12):2898-901. doi: 10.1681/ASN.2015030245. Epub 2015 Apr 29. J Am Soc Nephrol. 2015. PMID: 25925686 Free PMC article. No abstract available.
References
-
- Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML, Leichtman AB: Immunosuppression: Evolution in practice and trends, 1994-2004. Am J Transplant 6: 1111–1131, 2006 - PubMed
-
- Menon MC, Murphy B: Maintenance immunosuppression in renal transplantation. Curr Opin Pharmacol 13: 662–671, 2013 - PubMed
-
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000 - PubMed
-
- Meier-Kriesche HU, Schold JD, Kaplan B: Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 4: 1289–1295, 2004 - PubMed
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B: Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4: 378–383, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

