Collagen VI Is Upregulated in COPD and Serves Both as an Adhesive Target and a Bactericidal Barrier for Moraxella catarrhalis
- PMID: 25925694
- PMCID: PMC6738813
- DOI: 10.1159/000381213
Collagen VI Is Upregulated in COPD and Serves Both as an Adhesive Target and a Bactericidal Barrier for Moraxella catarrhalis
Abstract
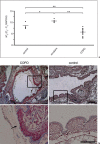
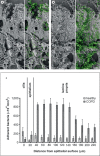
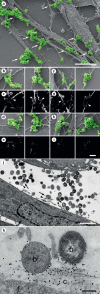
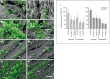
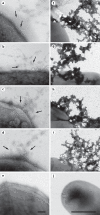
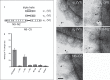
Moraxella catarrhalis is a Gram-negative human mucosal commensal and pathogen. It is a common cause of exacerbation in chronic obstructive pulmonary disease (COPD). During the process of infection, host colonization correlates with recognition of host molecular patterns. Importantly, in COPD patients with compromised epithelial integrity the underlying extracellular matrix is exposed and provides potential adhesive targets. Collagen VI is a ubiquitous fibrillar component in the airway mucosa and has been attributed both adhesive and killing properties against Gram-positive bacteria. However, less is known regarding Gram-negative microorganisms. Therefore, in the present study, the interaction of M. catarrhalis with collagen VI was characterized. We found that collagen VI is upregulated in the airways of COPD patients and exposed upon epithelial desquamation. Ex vivo, we inoculated airway biopsies and fibroblasts from COPD patients with M. catarrhalis. The bacteria specifically adhered to collagen VI-containing matrix fibrils. In vitro, purified collagen VI microfibrils bound to bacterial surface structures. The primary adhesion target was mapped to the collagen VI α2-chain. Upon exposure to collagen VI, bacteria were killed by membrane destabilization in physiological conditions. These previously unknown properties of collagen VI provide novel insights into the extracellular matrix innate immunity by quickly entrapping and killing pathogen intruders.
© 2015 S. Karger AG, Basel.
Figures

References
-
- Karalus R, Campagnari A. Moraxella catar rhalis: a review of an important human mucosal pathogen. Microbes Infect. 2000;2:547–559. - PubMed
-
- Aebi C. Moraxella catarrhalis - pathogen or commensal? Adv Exp Med Biol. 2011;697:107–116. - PubMed
-
- Stenfors LE, Raisanen S. Interaction between Streptococcus pneumoniae and Branhamella catarrhalis obtained from double-colonized, healthy nasopharynx and double-infected, diseased middle-ear cavity. Scand J Infect Dis. 1989;21:397–401. - PubMed
-
- Murphy TF, Parameswaran GI. Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis. 2009;49:124–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

