A randomized, open-label trial of iron isomaltoside 1000 (Monofer®) compared with iron sucrose (Venofer®) as maintenance therapy in haemodialysis patients
- PMID: 25925701
- PMCID: PMC4550440
- DOI: 10.1093/ndt/gfv096
A randomized, open-label trial of iron isomaltoside 1000 (Monofer®) compared with iron sucrose (Venofer®) as maintenance therapy in haemodialysis patients
Abstract
Background: Iron deficiency anaemia is common in patients with chronic kidney disease, and intravenous iron is the preferred treatment for those on haemodialysis. The aim of this trial was to compare the efficacy and safety of iron isomaltoside 1000 (Monofer®) with iron sucrose (Venofer®) in haemodialysis patients.
Methods: This was an open-label, randomized, multicentre, non-inferiority trial conducted in 351 haemodialysis subjects randomized 2:1 to either iron isomaltoside 1000 (Group A) or iron sucrose (Group B). Subjects in Group A were equally divided into A1 (500 mg single bolus injection) and A2 (500 mg split dose). Group B were also treated with 500 mg split dose. The primary end point was the proportion of subjects with haemoglobin (Hb) in the target range 9.5-12.5 g/dL at 6 weeks. Secondary outcome measures included haematology parameters and safety parameters.
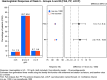
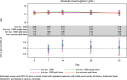
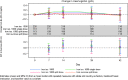
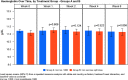
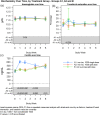
Results: A total of 351 subjects were enrolled. Both treatments showed similar efficacy with >82% of subjects with Hb in the target range (non-inferiority, P = 0.01). Similar results were found when comparing subgroups A1 and A2 with Group B. No statistical significant change in Hb concentration was found between any of the groups. There was a significant increase in ferritin from baseline to Weeks 1, 2 and 4 in Group A compared with Group B (Weeks 1 and 2: P < 0.001; Week 4: P = 0.002). There was a significant higher increase in reticulocyte count in Group A compared with Group B at Week 1 (P < 0.001). The frequency, type and severity of adverse events were similar.
Conclusions: Iron isomaltoside 1000 and iron sucrose have comparative efficacy in maintaining Hb concentrations in haemodialysis subjects and both preparations were well tolerated with a similar short-term safety profile.
Keywords: chronic kidney disease; iron isomaltoside 1000; iron treatment.
© The Author 2015. Published by Oxford University Press on behalf of ERA-EDTA.
Figures
References
-
- Senger JM, Weiss RJ. Hematologic and erythropoietin responses to iron dextran in the hemodialysis environment. ANNA J 1996; 23: 319–323 - PubMed
-
- Locatelli F, Aljama P, Barany P, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 2004; 19 (Suppl 2): ii1–ii47 - PubMed
-
- Albaramki J, Hodson EM, Craig JC, et al. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev 2012; 1: CD007857. - PubMed
-
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease . 3[1], http://www.kidney-international.org (17 December 2014, date last accessed) - PubMed
-
- Bhandari S, Brownjohn A, Turney J. Effective utilization of erythropoietin with intravenous iron therapy. J Clin Pharm Ther 1998; 23: 73–78 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical