Capecitabine and irinotecan with bevacizumab 2-weekly for metastatic colorectal cancer: the phase II AVAXIRI study
- PMID: 25925749
- PMCID: PMC4423590
- DOI: 10.1186/s12885-015-1293-y
Capecitabine and irinotecan with bevacizumab 2-weekly for metastatic colorectal cancer: the phase II AVAXIRI study
Abstract
Background: The optimal sequence of chemotherapeutic agents is not firmly established for the treatment of metastatic colorectal cancer (mCRC). This phase II multi-centre study investigated the efficacy and tolerability of a standard capecitabine plus irinotecan (XELIRI) regimen with bevacizumab in previously untreated patients with mCRC.
Methods: Patients received intravenous irinotecan 175 mg/m(2) on day 1 and oral capecitabine 1000 mg/m(2) (800 mg/m(2) for patients >65 years of age) twice daily on days 2-8, followed by a 1-week rest, and bevacizumab 5 mg/kg as an intravenous infusion on day 1 every 2 weeks.
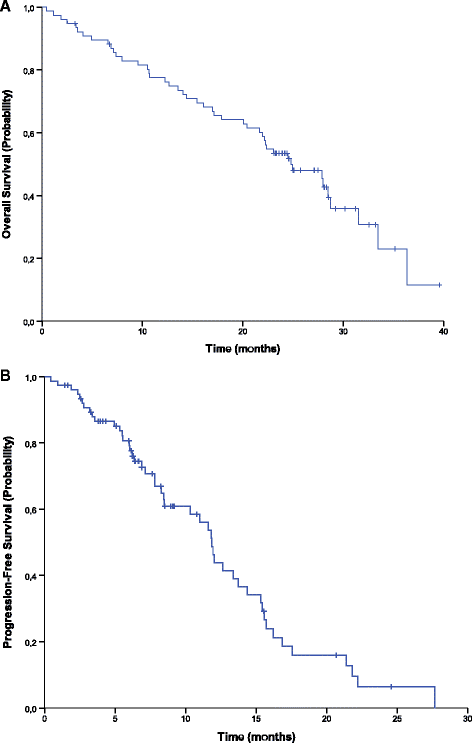
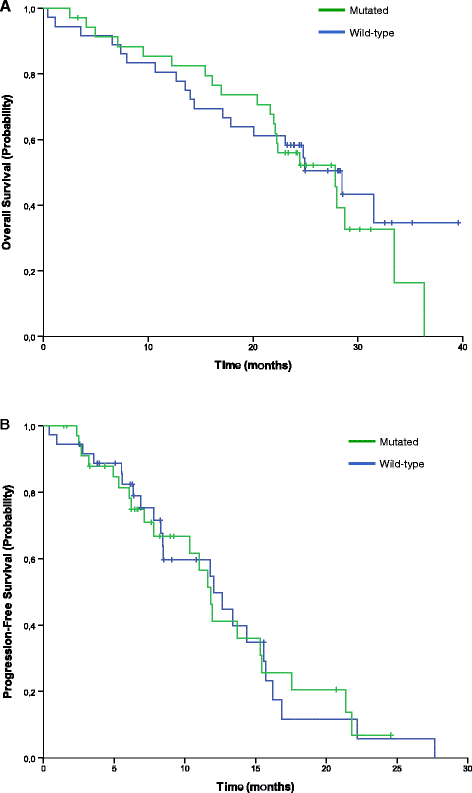
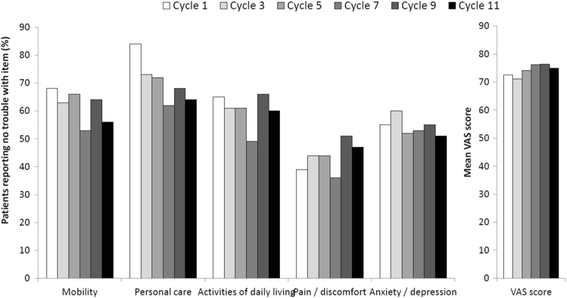
Results: Seventy-seven patients were included in the intention-to-treat and safety populations. Progression-free survival at 9 months was 61%. The overall response and disease control rates were 51% and 84%, respectively. Median progression-free and overall survival times were 11.9 and 24.8 months, respectively. 48 patients (62%) had at least one grade 3/4 adverse event, the most common being asthenia, diarrhoea and neutropenia. Quality of life varied little over the study period with mean visual analogue scale general health scores ranging from 71 to 76 over cycles 1-11.
Conclusion: Our study found irinotecan and capecitabine administered fortnightly with bevacizumab in patients with mCRC to be an effective and tolerable regimen.
Trial registration: clinicaltrials.gov identifier NCT00875771. Trial registration date: 04/02/2009.
Figures
References
-
- Ferlay J, Shin H, Bray F, Forman D, C Mathers, Parkin D. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. 2010. Lyon, France: International Agency for Research on Cancer. Available from: http://globocan.iarc.fr. Accessed on 10 November 2013.
-
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (2013) Colon cancer Version 3. Available at http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed on 6 March 2013.
-
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012;23:2479–516. doi: 10.1093/annonc/mds236. - DOI - PubMed
-
- Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:2013–9. doi: 10.1200/JCO.2007.14.9930. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

