Control of brain patterning by Engrailed paracrine transfer: a new function of the Pbx interaction domain
- PMID: 25926358
- PMCID: PMC4440920
- DOI: 10.1242/dev.114181
Control of brain patterning by Engrailed paracrine transfer: a new function of the Pbx interaction domain
Abstract
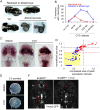
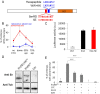
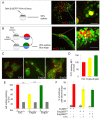
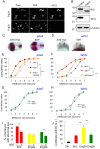
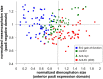
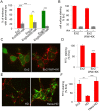
Homeoproteins of the Engrailed family are involved in the patterning of mesencephalic boundaries through a mechanism classically ascribed to their transcriptional functions. In light of recent reports on the paracrine activity of homeoproteins, including Engrailed, we asked whether Engrailed intercellular transfer was also involved in brain patterning and boundary formation. Using time-controlled activation of Engrailed combined with tools that block its transfer, we show that the positioning of the diencephalic-mesencephalic boundary (DMB) requires Engrailed paracrine activity. Both zebrafish Eng2a and Eng2b are competent for intercellular transfer in vivo, but only extracellular endogenous Eng2b, and not Eng2a, participates in DMB positioning. In addition, disruption of the Pbx-interacting motif in Engrailed, known to strongly reduce the gain-of-function phenotype, also downregulates Engrailed transfer, thus revealing an unsuspected participation of the Pbx interaction domain in this pathway.
Keywords: Diencephalic-mesencephalic boundary; Engrailed 2; Hexapeptide; Homeoprotein signalling.
© 2015. Published by The Company of Biologists Ltd.
Figures
References
-
- Araki I. and Nakamura H. (1999). Engrailed defines the position of dorsal di-mesencephalic boundary by repressing diencephalic fate. Development 126, 5127-5135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

