Driving sleep slow oscillations by auditory closed-loop stimulation-a self-limiting process
- PMID: 25926443
- PMCID: PMC4412888
- DOI: 10.1523/JNEUROSCI.3133-14.2015
Driving sleep slow oscillations by auditory closed-loop stimulation-a self-limiting process
Abstract
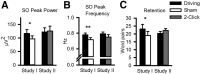
The <1 Hz EEG slow oscillation (SO) is a hallmark of slow-wave sleep (SWS) and is critically involved in sleep-associated memory formation. Previous studies showed that SOs and associated memory function can be effectively enhanced by closed-loop auditory stimulation, when clicks are presented in synchrony with upcoming SO up states. However, increasing SOs and synchronized excitability also bear the risk of emerging seizure activity, suggesting the presence of mechanisms in the healthy brain that counter developing hypersynchronicity during SOs. Here, we aimed to test the limits of driving SOs through closed-loop auditory stimulation in healthy humans. Study I tested a "Driving stimulation" protocol (vs "Sham") in which trains of clicks were presented in synchrony with SO up states basically as long as an ongoing SO train was identified on-line. Study II compared Driving stimulation with a "2-Click" protocol where the maximum of stimuli delivered in a train was limited to two clicks. Stimulation was applied during SWS in the first 210 min of nocturnal sleep. Before and after sleep declarative word-pair memories were tested. Compared with the Sham control, Driving stimulation prolonged SO trains and enhanced SO amplitudes, phase-locked spindle activity, and overnight retention of word pairs (all ps < 0.05). Importantly, effects of Driving stimulation did not exceed those of 2-Click stimulation (p > 0.180), indicating the presence of a mechanism preventing the development of hypersynchronicity during SO activity. Assessment of temporal dynamics revealed a rapidly fading phase-locked spindle activity during repetitive click stimulation, suggesting that spindle refractoriness contributes to this protective mechanism.
Keywords: auditory stimulation; closed-loop control; declarative memory consolidation; fast spindles; sleep; slow oscillations.
Copyright © 2015 Ngo, Miedema et al.
Figures




Comment in
-
A Dual Role for Sleep Spindles in Sleep-Dependent Memory Consolidation?J Neurosci. 2015 Sep 9;35(36):12328-30. doi: 10.1523/JNEUROSCI.2463-15.2015. J Neurosci. 2015. PMID: 26354902 Free PMC article. No abstract available.
References
-
- Ayoub A, Aumann D, Hörschelmann A, Kouchekmanesch A, Paul P, Born J, Marshall L. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. Sleep. 2013;36:905–911. doi: 10.5665/sleep.2722. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
