A network of visual motion-sensitive neurons for computing object position in an arthropod
- PMID: 25926445
- PMCID: PMC6605188
- DOI: 10.1523/JNEUROSCI.4667-14.2015
A network of visual motion-sensitive neurons for computing object position in an arthropod
Abstract
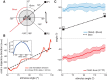
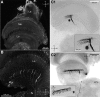
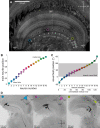
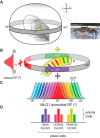
Highly active insects and crabs depend on visual motion information for detecting and tracking mates, prey, or predators, for which they require directional control systems containing internal maps of visual space. A neural map formed by large, motion-sensitive neurons implicated in processing panoramic flow is known to exist in an optic ganglion of the fly. However, an equivalent map for processing spatial positions of single objects has not been hitherto identified in any arthropod. Crabs can escape directly away from a visual threat wherever the stimulus is located in the 360° field of view. When tested in a walking simulator, the crab Neohelice granulata immediately adjusts its running direction after changes in the position of the visual danger stimulus smaller than 1°. Combining mass and single-cell staining with in vivo intracellular recording, we show that a particular class of motion-sensitive neurons of the crab's lobula that project to the midbrain, the monostratified lobula giants type 1 (MLG1), form a system of 16 retinotopically organized elements that map the 360° azimuthal space. The preference of these neurons for horizontally moving objects conforms the visual ecology of the crab's mudflat world. With a mean receptive field of 118°, MLG1s have a large superposition among neighboring elements. Our results suggest that the MLG1 system conveys information on object position as a population vector. Such computational code can enable the accurate directional control observed in the visually guided behaviors of crabs.
Keywords: cell ensemble; crab; escape direction; giant lobula neurons; insect; population coding.
Copyright © 2015 the authors 0270-6474/15/356654-13$15.00/0.
Figures




References
-
- Barnes WJP, Nalbach H-O. Eye movements in freely moving crabs: their sensory basis and possible role in flow-field analysis. Comparative Biochemistry and Physiology Part A: Physiology. 1993;104:675–693.
-
- Berón de Astrada M, Bengochea M, Medan V, Tomsic D. Regionalization in the eye of the grapsid crab Neohelice granulata (Chasmagnathus granulatus): variation of resolution and facet diameters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198:173–180. doi: 10.1007/s00359-011-0697-7. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
