Enhanced Firing in NTS Induced by Short-Term Sustained Hypoxia Is Modulated by Glia-Neuron Interaction
- PMID: 25926465
- PMCID: PMC6605179
- DOI: 10.1523/JNEUROSCI.4598-14.2015
Enhanced Firing in NTS Induced by Short-Term Sustained Hypoxia Is Modulated by Glia-Neuron Interaction
Abstract
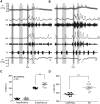
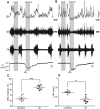
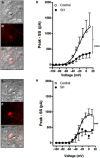
Humans ascending to high altitudes are submitted to sustained hypoxia (SH), activating peripheral chemoreflex with several autonomic and respiratory responses. Here we analyzed the effect of short-term SH (24 h, FIO210%) on the processing of cardiovascular and respiratory reflexes using an in situ preparation of rats. SH increased both the sympatho-inhibitory and bradycardiac components of baroreflex and the sympathetic and respiratory responses of peripheral chemoreflex. Electrophysiological properties and synaptic transmission in the nucleus tractus solitarius (NTS) neurons, the first synaptic station of afferents of baroreflexes and chemoreflexes, were evaluated using brainstem slices and whole-cell patch-clamp. The second-order NTS neurons were identified by previous application of fluorescent tracer onto carotid body for chemoreceptor afferents or onto aortic depressor nerve for baroreceptor afferents. SH increased the intrinsic excitability of NTS neurons. Delayed excitation, caused by A-type potassium current (IKA), was observed in most of NTS neurons from control rats. The IKA amplitude was higher in identified second-order NTS neurons from control than in SH rats. SH also blunted the astrocytic inhibition of IKA in NTS neurons and increased the synaptic transmission in response to afferent fibers stimulation. The frequency of spontaneous excitatory currents was also increased in neurons from SH rats, indicating that SH increased the neurotransmission by presynaptic mechanisms. Therefore, short-term SH changed the glia-neuron interaction, increasing the excitability and excitatory transmission of NTS neurons, which may contribute to the observed increase in the reflex sensitivity of baroreflex and chemoreflex in in situ preparation.
Keywords: NTS; astrocytes; firing; intrinsic properties; sustained hypoxia; synaptic transmission.
Copyright © 2015 the authors 0270-6474/15/356903-15$15.00/0.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
