The future of computer-aided sperm analysis
- PMID: 25926614
- PMCID: PMC4492043
- DOI: 10.4103/1008-682X.154312
The future of computer-aided sperm analysis
Abstract
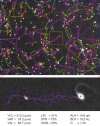
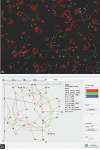
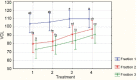
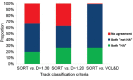
Computer-aided sperm analysis (CASA) technology was developed in the late 1980s for analyzing sperm movement characteristics or kinematics and has been highly successful in enabling this field of research. CASA has also been used with great success for measuring semen characteristics such as sperm concentration and proportions of progressive motility in many animal species, including wide application in domesticated animal production laboratories and reproductive toxicology. However, attempts to use CASA for human clinical semen analysis have largely met with poor success due to the inherent difficulties presented by many human semen samples caused by sperm clumping and heavy background debris that, until now, have precluded accurate digital image analysis. The authors review the improved capabilities of two modern CASA platforms (Hamilton Thorne CASA-II and Microptic SCA6) and consider their current and future applications with particular reference to directing our focus towards using this technology to assess functional rather than simple descriptive characteristics of spermatozoa. Specific requirements for validating CASA technology as a semi-automated system for human semen analysis are also provided, with particular reference to the accuracy and uncertainty of measurement expected of a robust medical laboratory test for implementation in clinical laboratories operating according to modern accreditation standards.
Figures

References
-
- Gray J. The movement of sea-urchin spermatozoa. J Exp Biol. 1955;32:775–801.
-
- Gray J. The movement of spermatozoa of the bull. J Exp Biol. 1958;35:96–108.
-
- Rikmenspoel R, van Herpen G. Photoelectric and cinematographic measurements of the motility of bull sperm cells. Phys Med Biol. 1957;2:54–63. - PubMed
-
- Rothschild L, Swann MM. The fertilization reaction in the sea-urchin egg; a propagated response to sperm attachment. J Exp Biol. 1949;26:164–76. 4 pl. - PubMed
-
- Rothschild L. A new method of measuring sperm speeds. Nature. 1953;171:512–3. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

