The Nectin-4/Afadin Protein Complex and Intercellular Membrane Pores Contribute to Rapid Spread of Measles Virus in Primary Human Airway Epithelia
- PMID: 25926640
- PMCID: PMC4473566
- DOI: 10.1128/JVI.00821-15
The Nectin-4/Afadin Protein Complex and Intercellular Membrane Pores Contribute to Rapid Spread of Measles Virus in Primary Human Airway Epithelia
Erratum in
-
Correction for Singh et al., The Nectin-4/Afadin Protein Complex and Intercellular Membrane Pores Contribute to Rapid Spread of Measles Virus in Primary Human Airway Epithelia.J Virol. 2016 Feb 26;90(6):3278. doi: 10.1128/JVI.03144-15. Print 2016 Mar. J Virol. 2016. PMID: 26921374 Free PMC article. No abstract available.
Abstract
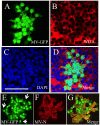
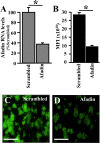
The discovery that measles virus (MV) uses the adherens junction protein nectin-4 as its epithelial receptor provides a new vantage point from which to characterize its rapid spread in the airway epithelium. We show here that in well-differentiated primary cultures of airway epithelial cells from human donors (HAE), MV infectious centers form rapidly and become larger than those of other respiratory pathogens: human respiratory syncytial virus, parainfluenza virus 5, and Sendai virus. While visible syncytia do not form after MV infection of HAE, the cytoplasm of an infected cell suddenly flows into an adjacent cell, as visualized through wild-type MV-expressed cytoplasmic green fluorescent protein (GFP). High-resolution video microscopy documents that GFP flows through openings that form on the lateral surfaces between columnar epithelial cells. To assess the relevance of the protein afadin, which connects nectin-4 to the actin cytoskeleton, we knocked down its mRNA. This resulted in more-limited infectious-center formation. We also generated a nectin-4 mutant without the afadin-binding site in its cytoplasmic tail. This mutant was less effective than wild-type human nectin-4 at promoting MV infection in primary cultures of porcine airway epithelia. Thus, in airway epithelial cells, MV spread requires the nectin-4/afadin complex and is based on cytoplasm transfer between columnar cells. Since the viral membrane fusion apparatus may open the passages that allow cytoplasm transfer, we refer to them as intercellular membrane pores. Virus-induced intercellular pores may contribute to extremely efficient measles contagion by promoting the rapid spread of the virus through the upper respiratory epithelium.
Importance: Measles virus (MV), while targeted for eradication, still causes about 120,000 deaths per year worldwide. The recent reemergence of measles in insufficiently vaccinated populations in Europe and North America reminds us that measles is extremely contagious, but the processes favoring its spread in the respiratory epithelium remain poorly defined. Here we characterize wild-type MV spread in well-differentiated primary cultures of human airway epithelial cells. We observed that viral infection promotes the flow of cytoplasmic contents from infected to proximal uninfected columnar epithelial cells. Cytoplasm flows through openings that form on the lateral surfaces. Infectious-center growth is facilitated by afadin, a protein connecting the adherens junction and the actin cytoskeleton. The viral fusion apparatus may open intercellular pores, and the cytoskeleton may stabilize them. Rapid homogenization of cytoplasmic contents in epithelial infectious centers may favor rapid spread and contribute to the extremely contagious nature of measles.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Chen SY, Anderson S, Kutty PK, Lugo F, McDonald M, Rota PA, Ortega-Sanchez IR, Komatsu K, Armstrong GL, Sunenshine R, Seward JF. 2011. Health care-associated measles outbreak in the United States after an importation: challenges and economic impact. J Infect Dis 203:1517–1525. doi:10.1093/infdis/jir115. - DOI - PubMed
-
- Ferreira CS, Frenzke M, Leonard VH, Welstead GG, Richardson CD, Cattaneo R. 2010. Measles virus infection of alveolar macrophages and dendritic cells precedes spread to lymphatic organs in transgenic mice expressing human signaling lymphocytic activation molecule (SLAM, CD150). J Virol 84:3033–3042. doi:10.1128/JVI.01559-09. - DOI - PMC - PubMed
-
- Lemon K, de Vries RD, Mesman AW, McQuaid S, van Amerongen G, Yuksel S, Ludlow M, Rennick LJ, Kuiken T, Rima BK, Geijtenbeek TB, Osterhaus AD, Duprex WP, de Swart RL. 2011. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog 7:e1001263. doi:10.1371/journal.ppat.1001263. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

