Mature cystic fibrosis airway neutrophils suppress T cell function: evidence for a role of arginase 1 but not programmed death-ligand 1
- PMID: 25926674
- PMCID: PMC4433848
- DOI: 10.4049/jimmunol.1500312
Mature cystic fibrosis airway neutrophils suppress T cell function: evidence for a role of arginase 1 but not programmed death-ligand 1
Abstract
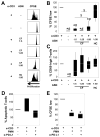
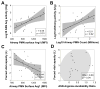
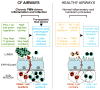
Bacteria colonize cystic fibrosis (CF) airways, and although T cells with appropriate Ag specificity are present in draining lymph nodes, they are conspicuously absent from the lumen. To account for this absence, we hypothesized that polymorphonuclear neutrophils (PMNs), recruited massively into the CF airway lumen and actively exocytosing primary granules, also suppress T cell function therein. Programmed death-ligand 1 (PD-L1), which exerts T cell suppression at a late step, was expressed bimodally on CF airway PMNs, delineating PD-L1(hi) and PD-L1(lo) subsets, whereas healthy control (HC) airway PMNs were uniformly PD-L1(hi). Blood PMNs incubated in CF airway fluid lost PD-L1 over time; in coculture, Ab blockade of PD-L1 failed to inhibit the suppression of T cell proliferation by CF airway PMNs. In contrast with PD-L1, arginase 1 (Arg1), which exerts T cell suppression at an early step, was uniformly high on CF and HC airway PMNs. However, arginase activity was high in CF airway fluid and minimal in HC airway fluid, consistent with the fact that Arg1 activation requires primary granule exocytosis, which occurs in CF, but not HC, airway PMNs. In addition, Arg1 expression on CF airway PMNs correlated negatively with lung function and positively with arginase activity in CF airway fluid. Finally, combined treatment with arginase inhibitor and arginine rescued the suppression of T cell proliferation by CF airway fluid. Thus, Arg1 and PD-L1 are dynamically modulated upon PMN migration into human airways, and, Arg1, but not PD-L1, contributes to early PMN-driven T cell suppression in CF, likely hampering resolution of infection and inflammation.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

