Cobicistat-boosted darunavir in HIV-1-infected adults: week 48 results of a Phase IIIb, open-label single-arm trial
- PMID: 25926858
- PMCID: PMC4413526
- DOI: 10.1186/1742-6405-11-39
Cobicistat-boosted darunavir in HIV-1-infected adults: week 48 results of a Phase IIIb, open-label single-arm trial
Abstract
Background: Cobicistat is an alternative pharmacoenhancer to ritonavir. In healthy volunteers, darunavir exposure was comparable when darunavir 800 mg once daily was co-administered with cobicistat 150 mg once daily (as single agents or a fixed-dose combination) vs. with ritonavir 100 mg once daily.
Methods: This 48-week, Phase IIIb, single-arm, US multicenter study (NCT01440569) evaluated safety, efficacy and pharmacokinetics of darunavir/cobicistat 800/150 mg once daily (as single agents) plus two investigator-selected nucleoside/tide reverse transcriptase inhibitors (N[t]RTIs) in HIV-1-infected adults. Patients had no darunavir resistance-associated mutations (RAMs), plasma viral load (VL) ≥1000 HIV-1 RNA copies/ml, eGFR ≥80 ml/min and genotypic sensitivity to the two N[t]RTIs. The primary endpoint was any treatment-emergent grade 3 or 4 adverse events (AEs) through Week 24.
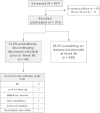
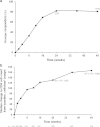
Results: The majority of the 313 intent-to-treat patients were treatment-naïve (295/313; 94%), male (89%), White (60%) and received a tenofovir-based regimen (99%). Median baseline VL and CD4(+) count overall were 4.8 log10 HIV-1 RNA copies/ml and 361 cells/mm(3), respectively. Overall, 86% of patients (268/313) completed the study. The majority of discontinuations were for AEs (15/313; 5%). The incidence of treatment-emergent grade 3 or 4 AEs regardless of causality was 6% through Week 24 and 8% through Week 48. Most common AEs through Week 48 were diarrhea (27%) and nausea (23%), which were grade 1 or 2 in severity. Week 48 virologic response rates (% with VL <50 HIV-1 RNA copies/ml; Snapshot analysis) were 81% overall and 83% in treatment-naïve patients; median increases in CD4(+) count at 48 weeks were 167 and 169 cells/mm(3), respectively. Of 15/313 patients who met the criteria for resistance analysis, one developed a darunavir RAM as a mixture with wild-type (I84I/V), without phenotypic resistance to darunavir. The mean population pharmacokinetic-derived darunavir areas under the plasma concentration-time curve were 102,000 overall and 100,620 ng•h/ml in treatment-naïve patients. No clinically relevant relationships were seen between darunavir exposure and virologic response, AEs or laboratory parameters.
Conclusion: Darunavir/cobicistat 800/150 mg once daily was generally well tolerated through Week 48, with no new safety concerns. Pharmacokinetics, virologic and immunologic responses for darunavir/cobicistat were similar to previous data for darunavir/ritonavir 800/100 mg once daily.
Keywords: Cobicistat; Darunavir; Efficacy; Pharmacokinetics; Safety; Virology.
Figures
References
-
- DHHS guidelines: Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. [http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf]
-
- Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Günthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA. Antiretroviral treatment of adult HIV infection: 2012 Recommendations of the International Antiviral Society–USA Panel. JAMA. 2012;308:387–402. - PubMed
-
- Williams I, Churchill D, Anderson J, Boffito M, Bower M, Cairns G, Cwynarski K, Edwards S, Fidler S, Fisher M, Freedman A, Geretti AM, Gilleece Y, Horne R, Johnson M, Khoo S, Leen C, Marshall N, Nelson M, Orkin C, Paton N, Phillips A, Post F, Pozniak A, Sabin C, Trevelion R, Ustianowski A, Walsh J, Waters L, Wilkins E, Winston A, Youle M. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012. HIV Med. 2012;13(Suppl. 2):1–85. - PubMed
-
- EACS: European guidelines for treatment of HIV infected adults in Europe. Version 7.02, updated June 2014. [http://www.eacsociety.org/Portals/0/140601_EACS%20EN7.02.pdf]
-
- Ortiz R, Dejesus E, Khanlou H, Voronin E, van Lunzen J, Andrade-Villanueva J, Fourie J, De Meyer S, De Pauw M, Lefebvre E, Vangeneugden T, Spinosa-Guzman S. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22:1389–1397. doi: 10.1097/QAD.0b013e32830285fb. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

