Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure
- PMID: 25927031
- PMCID: PMC4396194
- DOI: 10.3389/fonc.2015.00090
Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure
Abstract
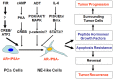
Neuroendocrine differentiation (NED) in prostate cancer is a well-recognized phenotypic change by which prostate cancer cells transdifferentiate into neuroendocrine-like (NE-like) cells. NE-like cells lack the expression of androgen receptor and prostate specific antigen, and are resistant to treatments. In addition, NE-like cells secrete peptide hormones and growth factors to support the growth of surrounding tumor cells in a paracrine manner. Accumulated evidence has suggested that NED is associated with disease progression and poor prognosis. The importance of NED in prostate cancer progression and therapeutic response is further supported by the fact that therapeutic agents, including androgen-deprivation therapy, chemotherapeutic agents, and radiotherapy, also induce NED. We will review the work supporting the overall hypothesis that therapy-induced NED is a mechanism of resistance to treatments, as well as discuss the relationship between therapy-induced NED and therapy-induced senescence, epithelial-to-mesenchymal transition, and cancer stem cells. Furthermore, we will use radiation-induced NED as a model to explore several NED-based targeting strategies for development of novel therapeutics. Finally, we propose future studies that will specifically address therapy-induced NED in the hope that a better treatment regimen for prostate cancer can be developed.
Keywords: ATF2; CREB; EMT; cancer stem cell; neuroendocrine differentiation; prostate cancer; radiosensitization; radiotherapy.
Figures


References
-
- D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA (1998) 280(11):969–74.10.1001/jama.280.11.969 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

