Nitric oxide donors for cervical ripening in first-trimester surgical abortion
- PMID: 25927092
- PMCID: PMC10961159
- DOI: 10.1002/14651858.CD007444.pub4
Nitric oxide donors for cervical ripening in first-trimester surgical abortion
Abstract
Background: Cervical priming before first-trimester surgical abortion is recommended in certain groups of women. Nitric oxide (NO) donors induce cervical ripening without uterine contractions, but the efficacy and side effects are of concern.
Objectives: To evaluate NO donors for cervical ripening before first-trimester surgical abortion, in terms of efficacy, side effects, and reduction of complications.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and POPLINE. We also searched reference lists of retrieved papers. We contacted experts in the field for information on both published and unpublished trials.
Selection criteria: Randomised controlled trials comparing NO donors alone or in combination with other methods for cervical ripening in first-trimester surgical abortion.
Data collection and analysis: Two review authors independently selected and extracted the data onto a data extraction form. We processed the data using Review Manager (RevMan 5) software.
Main results: We included 9 studies involving 766 participants. There were no serious complications (infection requiring antibiotic treatment, blood transfusion, complications requiring unintended operation, cervical injury, uterine perforation, death or serious morbidity) in the included trials.NO donors were more effective in cervical ripening when compared with placebo or no treatment. Baseline cervical dilatation before the procedure was higher in NO donors group (mean difference (MD) 0.30, 95% confidence interval (CI) 0.01 to 0.58) The cumulative force required to dilate the cervix to 8 mm (MD -4.29, 95% CI -9.92 to 1.35), headache (risk ratio (RR) 1.73, 95% CI 0.86 to 3.46), abdominal pain (RR 0.87, 95% CI 0.50 to 1.50), or patient satisfaction (RR 0.95, 95% CI 0.84 to 1.07) were not different. More nausea and vomiting occurred in the women who received a NO donor (RR 2.62, 95% CI 1.07 to 6.45).NO donors were inferior to prostaglandins for cervical ripening. The cumulative force required to dilate the cervix to 8 mm to 9 mm was higher (MD 13.12, 95% CI 9.72 to 16.52), and baseline cervical dilatation was less (MD -0.73, 95% CI -1.01 to -0.45) in the NO donor group. However, the probability of dilation greater than 8 mm at three hours was higher in the NO donor group (RR 6.67, 95% CI 2.21 to 20.09). Side effects including headache (RR 5.13, 95% CI 3.29 to 8.00), palpitation (RR 3.43, 95% CI 1.64 to 7.15), dizziness (RR 3.29, 95% CI 1.46 to 7.41), and intraoperative blood loss (MD 33.59 ml, 95% CI 24.50 to 42.67) were also higher. However, abdominal pain (RR 0.33, 95% CI 0.25 to 0.44) and vaginal bleeding (RR 0.14, 95% CI 0.07 to 0.27) were less in the NO donor group. No difference for nausea/vomiting in both groups(RR 1.17, 95% CI 0.94 to 1.46). Patient satisfaction was not different.One trial compared a NO donor with a NO donor plus prostaglandin. The cumulative force required to dilate the cervix to 8 mm was higher (MD 14.50, 95% CI 0.50 to 28.50) in the NO donor group. There was no difference in headache (RR 0.88, 95% CI 0.38 to 2.00), abdominal pain (RR 0.14, 95% CI 0.02 to 1.07), or intraoperative blood loss (MD -50, 95% CI -164.19 to 64.19).
Authors' conclusions: NO donors are superior to placebo or no treatment, but inferior to prostaglandins for first-trimester cervical ripening, and associated with more side effects.
Conflict of interest statement
None known.
Figures
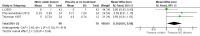
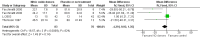
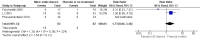
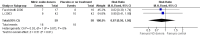
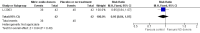
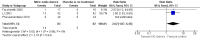
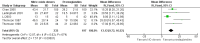
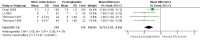
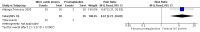
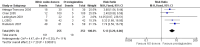
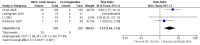

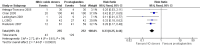

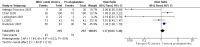
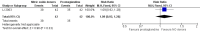
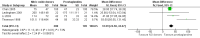
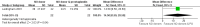
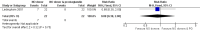
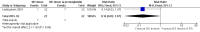
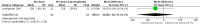





















Update of
-
Nitric oxide donors for cervical ripening in first-trimester surgical abortion.Cochrane Database Syst Rev. 2011 Dec 7;(12):CD007444. doi: 10.1002/14651858.CD007444.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Feb 25;(2):CD007444. doi: 10.1002/14651858.CD007444.pub4. PMID: 22161413 Updated.
References
References to studies included in this review
Arteaga‐Troncoso 2005 {published data only}
-
- Arteaga‐Troncoso G, Villegas‐Alvarado A, Belmont‐Gomez A, Martinez‐Herrera FJ, Villagrana‐Zesati R, Guerra‐Infante F. Intracervical application of the nitric oxide donor isosorbide dinitrate for induction of cervical ripening: a randomised controlled trial to determine clinical efficacy and safety prior to first trimester surgical evacuation of retained products of conception. BJOG: an International Journal of Obstetrics and Gynaecology 2005;112(12):1615‐9. - PubMed
Chan 2005 {published data only}
-
- Chan CCW, Tang OS, Ng EHY, Ho PC. Intracervical sodium nitroprusside versus vaginal misoprostol in first trimester surgical termination of pregnancy: a randomized double‐blinded controlled trial. Human Reproduction 2005;20(3):829‐33. - PubMed
Facchinetti 2000 {published data only}
-
- Facchinetti F, Piccinini F, Volpe A. Chemical ripening of the cervix with intracervical application of sodium nitroprusside: a randomized controlled trial. Human Reproduction 2000;15(10):2224‐7. - PubMed
Ledingham 2001 {published data only}
-
- Ledingham MA, Thomson AJ, Lunan CB, Greer IA, Norman JE. A comparison of isosorbide mononitrate, misoprostol and combination therapy for first trimester pre‐operative cervical ripening: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 2001;108(3):276‐80. - PubMed
Li 2003 {published data only}
-
- Li CFI, Chan CWC, Ho PC. A comparison of isosorbide mononitrate and misoprostol cervical ripening before suction evacuation. Obstetrics and Gynecology 2003;102(3):583‐8. - PubMed
Phusaanantakul 2010 {published data only (unpublished sought but not used)}
-
- Phusaanantakul P, Promsonthi P, Chanrachakul B. Effect of isosorbide mononitrate for cervical ripening before surgical termination of pregnancy in the first trimester. International Journal of Gynaecology Obstetrics 2010;110(2):145‐8. - PubMed
Radulovic 2007 {published data only}
-
- Radulovic N, Norstrom A, Ekerhovd E. Outpatient cervical ripening before first‐trimester surgical abortion: a comparison between misoprostol and isosorbide mononitrate. Acta Obstetricia et Gynecologica 2007;86(3):344‐8. - PubMed
Thomson 1997 {published data only}
-
- Thomson AJ, Lunan CB, Cameron AD, Cameron IT, Greer IA, Norman JE. Nitric oxide donors induce ripening of the human uterine cervix: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 1997;104(9):1054‐7. - PubMed
Thomson 1998 {published data only}
-
- Thomson AJ, Lunan CB, Ledingham M, Howat RCL, Cameron IT, Greer IA, et al. Randomised trial of nitric oxide donor versus prostaglandin for cervical ripening before first‐trimester termination of pregnancy. The Lancet 1998;352(9134):1093‐6. - PubMed
References to studies excluded from this review
Chen 2008 {published data only}
-
- Chen FCK, Bergann A, Krosse J, Merholz A, David M. Isosorbide mononitrate vaginal gel versus misoprostol vaginal gel versus Dilapan‐S for cervical ripening before first trimester curettage. European Journal of Obstetrics & Gynecology and Reproductive Biology 2008;138(2):176‐9. - PubMed
David 2003 {published data only}
-
- David M, Chen FCK, Lichtenegger W. NO‐donor nitroglycerin versus prostaglandin gemeprost for cervical ripening in first trimester missed abortion. International Journal of Gynecology and Obstetrics 2003;83(1):71‐2. - PubMed
David 2005 {published data only}
-
- David M, Chen FCK. Comparison of isosorbide mononitrate (Mono Mack) and misoprostol (Cytotec) for cervical ripening in the first trimester missed abortion. Archives of Gynecology and Obstetrics 2005;273(3):144‐5. - PubMed
Duhan 2011 {published data only}
-
- Duhan N, Gupta S, Dahiya K, Sirohiwal D, Rohilla S. Comparison of isosorbide mononitrate and misoprostol for cervical ripening in termination of pregnancy between 8 and 12 weeks: a randomized controlled trial. Archives of Gynecology and Obstetrics 2011;283(6):1245‐8. - PubMed
Additional references
Allen 2007
-
- Allen RH, Goldberg AB, Board of Society of Family Planning. Cervical dilation before first‐trimester surgical abortion (<14 weeks' gestation). SFP Guideline 20071. Contraception 2007;76(2):139‐56. - PubMed
Ekerhovd 2003
-
- Ekerhovd E, Radulovic N, Norström A. Gemeprost versus misoprostol for cervical priming before first‐trimester abortion: a randomized controlled trial. Obstetrics and Gynecology 2003;101(4):722‐5. - PubMed
Grimes 1984
-
- Grimes DA, Schulz KF, Cates WJ Jr. Prevention of uterine perforation during curettage abortion. JAMA 1984;251(16):2108‐11. - PubMed
Hakim‐Elahi 1990
-
- Hakim‐Elahi E, Tovell HM, Burnhill MS. Complications of first‐trimester abortion: a report of 170,000 cases. Obstetrics and Gynecology 1990;76(1):129‐35. - PubMed
Hayashi 1993
-
- Hayashi RH. Spontaneous and induced cervical ripening. Natural dilation and effacement process and current cervical ripening techniques. Journal of Reproductive Medicine 1993;38(1 Suppl):66‐72. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ledingham 2000
-
- Ledingham MA, Thomson AJ, Young A, Macara LM, Greer IA, Norman JE. Changes in the expression of nitric oxide synthase in the human uterine cervix during pregnancy and parturition. Molecular Human Reproduction 2000;6(11):1041‐8. - PubMed
RCOG 2011
-
- Royal College of Obstetricians and Gynaecologists. The care of women requesting induced abortion. Evidence‐based guideline No.7. London: RCOG Press, 2011.
RevMan 5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 1983
-
- Schulz KF, Grimes DA, Cates W Jr. Measures to prevent cervical injury during suction curettage abortion. The Lancet 1983;1(8335):1182‐5. - PubMed
Sharma 2005
-
- Sharma S, Refaey H, Stafford M, Purkayastha S, Parry M, Axby H. Oral versus vaginal misoprostol administered one hour before surgical termination of pregnancy: a randomised controlled trial. BJOG: An International Journal of Obstetrics and Gynaecology 2005;112(4):456‐60. - PubMed
Tschugguel 1999
-
- Tschugguel W, Schneeberger C, Lass H, Stonek F, Zaghlula MB, Czerwenka K, et al. Human cervical ripening is associated with an increase in cervical inducible nitric oxide synthase expression. Biology of Reproduction 1999;60(6):1367‐72. - PubMed
WHO 2012
-
- World Health Organization. Safe abortion: technical and policy guidance for health systems. http://apps.who.int/iris/bitstream/10665/70914/1/9789241548434_eng.pdf (accessed 16 October 2014). - PubMed
Zhou 2002
-
- Zhou W, Nielsen GL, Møller M, Olsen J. Short‐term complications after surgically induced abortions: a register‐based study of 56 117 abortions. Acta Obstetricia et Gynecologica Scandinavica 2002;81(4):331‐6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

