Progesterone metabolites regulate induction, growth, and suppression of estrogen- and progesterone receptor-negative human breast cell tumors
- PMID: 25927181
- PMCID: PMC3706910
- DOI: 10.1186/bcr3422
Progesterone metabolites regulate induction, growth, and suppression of estrogen- and progesterone receptor-negative human breast cell tumors
Abstract
Introduction: Of the nearly 1.4 million new cases of breast cancer diagnosed each year, a large proportion is characterized as hormone receptor negative, lacking estrogen receptors (ER) and/or progesterone receptors (PR). Patients with receptor-negative tumors do not respond to current steroid hormone-based therapies and generally have significantly higher risk of recurrence and mortality compared with patients with tumors that are ER- and/or PR-positive. Previous in vitro studies had shown that the progesterone metabolites, 5α-dihydroprogesterone (5αP) and 3α-dihydroprogesterone (3αHP), respectively, exhibit procancer and anticancer effects on receptor-negative human breast cell lines. Here in vivo studies were conducted to investigate the ability of 5αP and 3αHP to control initiation, growth, and regression of ER/PR-negative human breast cell tumors.
Methods: ER/PR-negative human breast cells (MDA-MB-231) were implanted into mammary fat pads of immunosuppressed mice, and the effects of 5αP and 3αHP treatments on tumor initiation, growth, suppression/regression, and histopathology were assessed in five separate experiments. Specific radioimmunoassays and gas chromatography-mass spectrometry were used to measure 5αP, 3αHP, and progesterone in mouse serum and tumors.
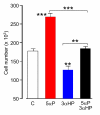
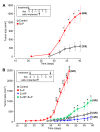
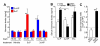
Results: Onset and growth of ER/PR-negative human breast cell tumors were significantly stimulated by 5αP and inhibited by 3αHP. When both hormones were applied simultaneously, the stimulatory effects of 5αP were abrogated by the inhibitory effects of 3αHP and vice versa. Treatment with 3αHP subsequent to 5αP-induced tumor initiation resulted in suppression of further tumorigenesis and regression of existing tumors. The levels of 5αP in tumors, regardless of treatment, were about 10-fold higher than the levels of 3αHP, and the 5αP:3αHP ratios were about fivefold higher than in serum, indicating significant changes in endogenous synthesis of these hormones in tumorous breast tissues.
Conclusions: The studies showed that estrogen/progesterone-insensitive breast tumors are sensitive to, and controlled by, the progesterone metabolites 5αP and 3αHP. Tumorigenesis of ER/PR-negative breast cells is significantly enhanced by 5αP and suppressed by 3αHP, the outcome depending on the relative concentrations of these two hormones in the microenvironment in the breast regions. The findings show that the production of 5αP greatly exceeds that of 3αHP in ER/PR-negative tumors and that treatment with 3αHP can effectively block tumorigenesis and cause existing tumors to regress. The results provide the first hormonal theory to explain tumorigenesis of ER/PR-negative breast tissues and support the hypothesis that a high 3αHP-to-5αP concentration ratio in the microenvironment may foster normalcy in noncancerous breast regions. The findings suggest new diagnostics based on the relative levels of these hormones and new approaches to prevention and treatment of breast cancers based on regulating the levels and action mechanisms of anti- and pro-cancer progesterone metabolites.
Figures





Similar articles
-
Opposing actions of the progesterone metabolites, 5alpha-dihydroprogesterone (5alphaP) and 3alpha-dihydroprogesterone (3alphaHP) on mitosis, apoptosis, and expression of Bcl-2, Bax and p21 in human breast cell lines.J Steroid Biochem Mol Biol. 2010 Jan;118(1-2):125-32. doi: 10.1016/j.jsbmb.2009.11.005. Epub 2009 Nov 17. J Steroid Biochem Mol Biol. 2010. PMID: 19931389
-
Membrane 5alpha-pregnane-3,20-dione (5alphaP) receptors in MCF-7 and MCF-10A breast cancer cells are up-regulated by estradiol and 5alphaP and down-regulated by the progesterone metabolites, 3alpha-dihydroprogesterone and 20alpha-dihydroprogesterone, with associated changes in cell proliferation and detachment.J Steroid Biochem Mol Biol. 2005 Nov;97(3):278-88. doi: 10.1016/j.jsbmb.2005.05.014. Epub 2005 Sep 9. J Steroid Biochem Mol Biol. 2005. PMID: 16154741
-
Plasma membrane receptors for the cancer-regulating progesterone metabolites, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-4-pregnen-20-one in MCF-7 breast cancer cells.Biochem Biophys Res Commun. 2000 Jun 16;272(3):731-7. doi: 10.1006/bbrc.2000.2847. Biochem Biophys Res Commun. 2000. PMID: 10860824
-
Progesterone metabolites in breast cancer.Endocr Relat Cancer. 2006 Sep;13(3):717-38. doi: 10.1677/erc.1.01010. Endocr Relat Cancer. 2006. PMID: 16954427 Review.
-
Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy.J Clin Oncol. 2005 Oct 20;23(30):7721-35. doi: 10.1200/JCO.2005.09.004. J Clin Oncol. 2005. PMID: 16234531 Review.
Cited by
-
Rewiring of the Endocrine Network in Triple-Negative Breast Cancer.Front Oncol. 2022 Jun 30;12:830894. doi: 10.3389/fonc.2022.830894. eCollection 2022. Front Oncol. 2022. PMID: 35847875 Free PMC article. Review.
-
Reproducibility of an assay to measure serum progesterone metabolites that may be related to breast cancer risk using liquid chromatography-tandem mass spectrometry.Horm Mol Biol Clin Investig. 2015 Sep;23(3):79-84. doi: 10.1515/hmbci-2015-0026. Horm Mol Biol Clin Investig. 2015. PMID: 26353176 Free PMC article.
-
Progesterone and Breast Cancer.Endocr Rev. 2020 Apr 1;41(2):320-44. doi: 10.1210/endrev/bnz001. Endocr Rev. 2020. PMID: 31512725 Free PMC article. Review.
-
Lentivirus-mediated CDglyTK gene-modified free flaps by intra-artery perfusion show targeted therapeutic efficacy in rat model of breast cancer.BMC Cancer. 2019 Sep 14;19(1):921. doi: 10.1186/s12885-019-6111-5. BMC Cancer. 2019. PMID: 31521130 Free PMC article.
-
Differential metabolism of clinically-relevant progestogens in cell lines and tissue: Implications for biological mechanisms.J Steroid Biochem Mol Biol. 2019 May;189:145-153. doi: 10.1016/j.jsbmb.2019.02.010. Epub 2019 Feb 26. J Steroid Biochem Mol Biol. 2019. PMID: 30822501 Free PMC article.
References
-
- American Cancer Society. Global Cancer Facts & Figures. 2. Atlanta, American Cancer Society; 2011. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume...
-
- McGuire WL, Osborne CK, Clark GM, Knight WA III. Steroid hormone receptors and carcinoma of the breast. Am J Physiol. 1982;243:E99–E102. - PubMed
-
- Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, Syrjanen K. Hormone receptors as prognostic factors in female breast cancer. Ann Med. 1991;6:643–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

