Effects of age and acute ethanol on glutamatergic neurotransmission in the medial prefrontal cortex of freely moving rats using enzyme-based microelectrode amperometry
- PMID: 25927237
- PMCID: PMC4416039
- DOI: 10.1371/journal.pone.0125567
Effects of age and acute ethanol on glutamatergic neurotransmission in the medial prefrontal cortex of freely moving rats using enzyme-based microelectrode amperometry
Abstract
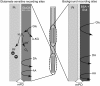
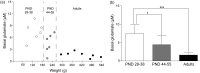
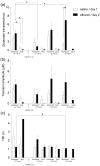
Ethanol abuse during adolescence may significantly alter development of the prefrontal cortex which continues to undergo structural remodeling into adulthood. Glutamatergic neurotransmission plays an important role during these brain maturation processes and is modulated by ethanol. In this study, we investigated glutamate dynamics in the medial prefrontal cortex of freely moving rats, using enzyme-based microelectrode amperometry. We analyzed the effects of an intraperitoneal ethanol injection (1 g/kg) on cortical glutamate levels in adolescent and adult rats. Notably, basal glutamate levels decreased with age and these levels were found to be significantly different between postnatal day (PND) 28-38 vs PND 44-55 (p<0.05) and PND 28-38 vs adult animals (p<0.001). We also observed spontaneous glutamate release (transients) throughout the recordings. The frequency of transients (per hour) was significantly higher in adolescent rats (PND 28-38 and PND 44-55) compared to those of adults. In adolescent rats, post-ethanol injection, the frequency of glutamate transients decreased within the first hour (p<0.05), it recovered slowly and in the third hour there was a significant rebound increase of the frequency (p<0.05). Our data demonstrate age-dependent differences in extracellular glutamate levels in the medial prefrontal cortex and suggest that acute ethanol injections have both inhibitory and excitatory effects in adolescent rats. These effects of ethanol on the prefrontal cortex may disturb its maturation and possibly limiting individuals´ control over addictive behaviors.
Conflict of interest statement
Figures




References
-
- deGraaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early human development 2006;82: 257–266. - PubMed
-
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24: 417–63. - PubMed
-
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. Journal of child psychology and psychiatry, and allied disciplines. 2006;47: 296–312. - PubMed
-
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283: 1923–7. - PubMed
-
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999; 402:421–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

