Phase I Clinical Trial of a Recombinant Blood Stage Vaccine Candidate for Plasmodium falciparum Malaria Based on MSP1 and EBA175
- PMID: 25927360
- PMCID: PMC4415778
- DOI: 10.1371/journal.pone.0117820
Phase I Clinical Trial of a Recombinant Blood Stage Vaccine Candidate for Plasmodium falciparum Malaria Based on MSP1 and EBA175
Erratum in
-
Correction: Phase I Clinical Trial of a Recombinant Blood Stage Vaccine Candidate for Plasmodium falciparum Malaria Based on MSP1 and EBA175.PLoS One. 2015 Sep 2;10(9):e0137816. doi: 10.1371/journal.pone.0137816. eCollection 2015. PLoS One. 2015. PMID: 26332825 Free PMC article.
Abstract
Background: A phase I randomised, controlled, single blind, dose escalation trial was conducted to evaluate safety and immunogenicity of JAIVAC-1, a recombinant blood stage vaccine candidate against Plasmodium falciparum malaria, composed of a physical mixture of two recombinant proteins, PfMSP-1(19), the 19 kD conserved, C-terminal region of PfMSP-1 and PfF2 the receptor-binding F2 domain of EBA175.
Method: Healthy malaria naïve Indian male subjects aged 18-45 years were recruited from the volunteer database of study site. Fifteen subjects in each cohort, randomised in a ratio of 2:1 and meeting the protocol specific eligibility criteria, were vaccinated either with three doses (10 μg, 25 μg and 50 μg of each antigen) of JAIVAC-1 formulated with adjuvant Montanide ISA 720 or with standard dosage of Hepatitis B vaccine. Each subject received the assigned vaccine in the deltoid muscle of the upper arms on Day 0, Day 28 and Day 180.
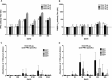
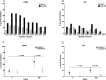
Results: JAIVAC-1 was well tolerated and no serious adverse event was observed. All JAIVAC-1 subjects sero-converted for PfF2 but elicited poor immune response to PfMSP-1(19). Dose-response relationship was observed between vaccine dose of PfF2 and antibody response. The antibodies against PfF2 were predominantly of IgG1 and IgG3 isotype. Sera from JAIVAC-1 subjects reacted with late schizonts in a punctate pattern in immunofluorescence assays. Purified IgG from JAIVAC-1 sera displayed significant growth inhibitory activity against Plasmodium falciparum CAMP strain.
Conclusion: Antigen PfF2 should be retained as a component of a recombinant malaria vaccine but PfMSP-1(19) construct needs to be optimised to improve its immunogenicity.
Trial registration: Clinical Trial Registry, India CTRI/2010/091/000301.
Conflict of interest statement
Figures

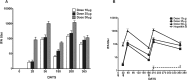
Similar articles
-
Immunogenicity of a chimeric Plasmodium falciparum merozoite surface protein vaccine in Aotus monkeys.Malar J. 2016 Mar 15;15:159. doi: 10.1186/s12936-016-1226-5. Malar J. 2016. PMID: 26975721 Free PMC article.
-
Comparative analysis of the profiles of IgG subclass-specific responses to Plasmodium falciparum apical membrane antigen-1 and merozoite surface protein-1 in naturally exposed individuals living in malaria hypoendemic settings, Iran.Malar J. 2015 Feb 5;14:58. doi: 10.1186/s12936-015-0547-0. Malar J. 2015. PMID: 25652589 Free PMC article.
-
Phase 1 safety and immunogenicity trial of the Plasmodium falciparum blood-stage malaria vaccine AMA1-C1/ISA 720 in Australian adults.Vaccine. 2010 Mar 2;28(10):2236-2242. doi: 10.1016/j.vaccine.2009.12.049. Epub 2010 Jan 4. Vaccine. 2010. PMID: 20051276 Free PMC article. Clinical Trial.
-
Antibodies and Plasmodium falciparum merozoites.Trends Parasitol. 2001 Apr;17(4):194-7. doi: 10.1016/s1471-4922(00)01946-2. Trends Parasitol. 2001. PMID: 11282510 Review.
-
The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria.Parasitology. 2009 Oct;136(12):1445-56. doi: 10.1017/S0031182009990515. Epub 2009 Jul 23. Parasitology. 2009. PMID: 19627632 Review.
Cited by
-
Development & licensing of first ever vaccine against malaria.Indian J Med Res. 2015 Dec;142(6):637-9. doi: 10.4103/0971-5916.174535. Indian J Med Res. 2015. PMID: 26831410 Free PMC article. No abstract available.
-
Correction: Phase I Clinical Trial of a Recombinant Blood Stage Vaccine Candidate for Plasmodium falciparum Malaria Based on MSP1 and EBA175.PLoS One. 2015 Sep 2;10(9):e0137816. doi: 10.1371/journal.pone.0137816. eCollection 2015. PLoS One. 2015. PMID: 26332825 Free PMC article.
-
Neutralizing and interfering human antibodies define the structural and mechanistic basis for antigenic diversion.Nat Commun. 2022 Oct 6;13(1):5888. doi: 10.1038/s41467-022-33336-3. Nat Commun. 2022. PMID: 36202833 Free PMC article.
-
Forest malaria and prospects for anti-malarial chemoprophylaxis among forest goers: findings from a qualitative study in Thailand.Malar J. 2022 Feb 14;21(1):47. doi: 10.1186/s12936-022-04070-4. Malar J. 2022. PMID: 35164759 Free PMC article.
-
Combating Human Viral Diseases: Will Plant-Based Vaccines Be the Answer?Vaccines (Basel). 2021 Jul 8;9(7):761. doi: 10.3390/vaccines9070761. Vaccines (Basel). 2021. PMID: 34358177 Free PMC article. Review.
References
-
- World Health Organization Media Centre: Factsheet on the World Malaria Report 2013 December 2013, Available: http://www.who.int/malaria/media/world_malaria_report_2013/en/. Accessed 2015 January 07.
-
- Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH (1994) Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum . Science 264: 1941–1944. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

