The shh signaling pathway is upregulated in multiple cell types in cortical ischemia and influences the outcome of stroke in an animal model
- PMID: 25927436
- PMCID: PMC4415811
- DOI: 10.1371/journal.pone.0124657
The shh signaling pathway is upregulated in multiple cell types in cortical ischemia and influences the outcome of stroke in an animal model
Abstract
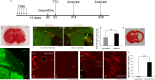
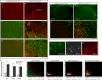
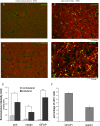
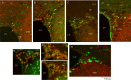
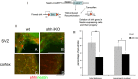
Recently the sonic hedgehog (shh) signaling pathway has been shown to play an important role in regulating repair and regenerative responses after brain injury, including ischemia. However, the precise cellular components that express and upregulate the shh gene and the cellular components that respond to shh signaling remain to be identified. In this study, using a distal MCA occlusion model, our data show that the shh signal is upregulated both at the cortical area near the injury site and in the adjacent striatum. Multiple cell types upregulate shh signaling in ischemic brain, including neurons, reactive astrocytes and nestin-expressing cells. The shh signaling pathway genes are also expressed in the neural stem cells (NSCs) niche in the subventricular zone (SVZ). Conditional deletion of the shh gene in nestin-expressing cells both at the SVZ niche and at the ischemic site lead to significantly more severe behavioral deficits in these shh iKO mice after cortical stroke, measured using an automated open field locomotion apparatus (Student's t-test, p<0.05). In contrast, animals given post-stroke treatment with the shh signaling agonist (SAG) demonstrated less deficits in behavioral function, compared to vehicle-treated mice. At 7 days after stroke, SAG-treated mice showed higher values in multiple horizontal movement parameters compared to vehicle treated mice (Student's t-test, p<0.05) whereas there were no differences in pre-stroke measurements, (Student's t-test, p>0.05). In summary, our data demonstrate that shh signaling plays critical and ongoing roles in response to ischemic injury and modulation of shh signaling in vivo alters the functional outcome after cortical ischemic injury.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

