Central presynaptic terminals are enriched in ATP but the majority lack mitochondria
- PMID: 25928229
- PMCID: PMC4416033
- DOI: 10.1371/journal.pone.0125185
Central presynaptic terminals are enriched in ATP but the majority lack mitochondria
Erratum in
-
Correction: Central Presynaptic Terminals Are Enriched in ATP but the Majority Lack Mitochondria.PLoS One. 2017 Jul 10;12(7):e0181140. doi: 10.1371/journal.pone.0181140. eCollection 2017. PLoS One. 2017. PMID: 28700683 Free PMC article.
Abstract
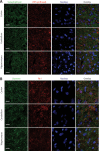
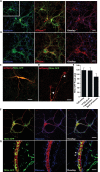
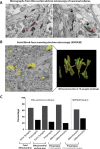
Synaptic neurotransmission is known to be an energy demanding process. At the presynapse, ATP is required for loading neurotransmitters into synaptic vesicles, for priming synaptic vesicles before release, and as a substrate for various kinases and ATPases. Although it is assumed that presynaptic sites usually harbor local mitochondria, which may serve as energy powerhouse to generate ATP as well as a presynaptic calcium depot, a clear role of presynaptic mitochondria in biochemical functioning of the presynapse is not well-defined. Besides a few synaptic subtypes like the mossy fibers and the Calyx of Held, most central presynaptic sites are either en passant or tiny axonal terminals that have little space to accommodate a large mitochondrion. Here, we have used imaging studies to demonstrate that mitochondrial antigens poorly co-localize with the synaptic vesicle clusters and active zone marker in the cerebral cortex, hippocampus and the cerebellum. Confocal imaging analysis on neuronal cultures revealed that most neuronal mitochondria are either somatic or distributed in the proximal part of major dendrites. A large number of synapses in culture are devoid of any mitochondria. Electron micrographs from neuronal cultures further confirm our finding that the majority of presynapses may not harbor resident mitochondria. We corroborated our ultrastructural findings using serial block face scanning electron microscopy (SBFSEM) and found that more than 60% of the presynaptic terminals lacked discernible mitochondria in the wild-type mice hippocampus. Biochemical fractionation of crude synaptosomes into mitochondria and pure synaptosomes also revealed a sparse presence of mitochondrial antigen at the presynaptic boutons. Despite a low abundance of mitochondria, the synaptosomal membranes were found to be highly enriched in ATP suggesting that the presynapse may possess alternative mechanism/s for concentrating ATP for its function. The potential mechanisms including local glycolysis and the possible roles of ATP-binding synaptic proteins such as synapsins, are discussed.
Conflict of interest statement
Figures


References
-
- Mink JW, Blumenschine RJ, Adams DB. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Physiol. 1981;241(3):R203–12. - PubMed
-
- Aiello LC, Wheeler P. The expensive tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol. 1995;36:199–221.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

