Exploring the perspectives and preferences for HTA across German healthcare stakeholders using a multi-criteria assessment of a pulmonary heart sensor as a case study
- PMID: 25928535
- PMCID: PMC4424515
- DOI: 10.1186/s12961-015-0011-1
Exploring the perspectives and preferences for HTA across German healthcare stakeholders using a multi-criteria assessment of a pulmonary heart sensor as a case study
Abstract
Background: Health technology assessment and healthcare decision-making are based on multiple criteria and evidence, and heterogeneous opinions of participating stakeholders. Multi-criteria decision analysis (MCDA) offers a potential framework to systematize this process and take different perspectives into account. The objectives of this study were to explore perspectives and preferences across German stakeholders when appraising healthcare interventions, using multi-criteria assessment of a heart pulmonary sensor as a case study.
Methods: An online survey of 100 German healthcare stakeholders was conducted using a comprehensive MCDA framework (EVIDEM V2.2). Participants were asked to provide i) relative weights for each criterion of the framework; ii) performance scores for a health pulmonary sensor, based on available data synthesized for each criterion; and iii) qualitative feedback on the consideration of contextual criteria. Normalized weights and scores were combined using a linear model to calculate a value estimate across different stakeholders. Differences across types of stakeholders were explored.
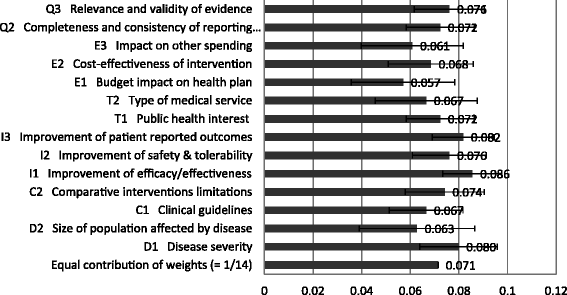
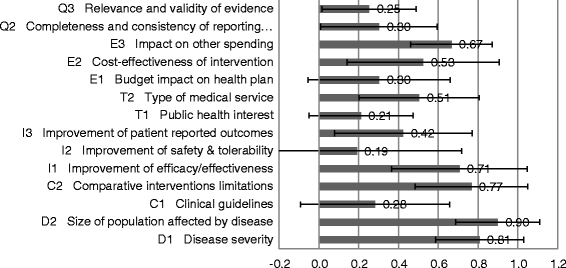
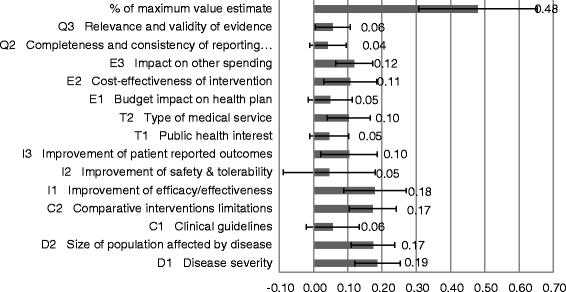
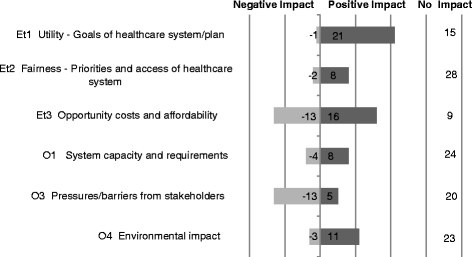
Results: The survey was completed by 54 participants. The most important criteria were efficacy, patient reported outcomes, disease severity, safety, and quality of evidence (relative weight >0.075 each). Compared to all participants, policymakers gave more weight to budget impact and quality of evidence. The quantitative appraisal of a pulmonary heart sensor revealed differences in scoring performance of this intervention at the criteria level between stakeholder groups. The highest value estimate of the sensor reached 0.68 (on a scale of 0 to 1, 1 representing maximum value) for industry representatives and the lowest value of 0.40 was reported for policymakers, compared to 0.48 for all participants. Participants indicated that most qualitative criteria should be considered and their impact on the quantitative appraisal was captured transparently.
Conclusions: The study identified important variations in perspectives across German stakeholders when appraising a healthcare intervention and revealed that MCDA can demonstrate the value of a specified technology for all participating stakeholders. Better understanding of these differences at the criteria level, in particular between policymakers and industry representatives, is important to focus innovation aligned with patient health and healthcare system values and constraints.
Figures
References
-
- Definition of HTA [http://www.eunethta.eu/about-us/faq#t287n73]
-
- Wahlster P, Scahill S, Garg S, Babar Z-U-D. Identifying stakeholder opinion regarding access to “high-cost medicines”: A systematic review of the literature. Central Eur J Med. 2014;9:513–27. doi: 10.2478/s11536-013-0286-y. - DOI
-
- Porter ME, Olmsted TE. Redefining Health Care: Creating Value-Based Competition on Results. Boston, Mass: Harvard Business School Press; 2006.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

