A cluster randomized trial of a multifaceted quality improvement intervention in Brazilian intensive care units: study protocol
- PMID: 25928627
- PMCID: PMC4342101
- DOI: 10.1186/s13012-014-0190-0
A cluster randomized trial of a multifaceted quality improvement intervention in Brazilian intensive care units: study protocol
Erratum in
-
Erratum to: A cluster randomized trial of a multifaceted quality improvement intervention in Brazilian intensive care units: study protocol.Implement Sci. 2015 Aug 13;10:116. doi: 10.1186/s13012-015-0288-z. Implement Sci. 2015. PMID: 26268555 Free PMC article. No abstract available.
Abstract
Background: The uptake of evidence-based therapies in the intensive care environment is suboptimal, particularly in limited-resource countries. Checklists, daily goal assessments, and clinician prompts may improve compliance with best practice processes of care and, in turn, improve clinical outcomes. However, the available evidence on the effectiveness of checklists is unreliable and inconclusive, and the mechanisms are poorly understood. We aim to evaluate whether the use of a multifaceted quality improvement intervention, including the use of a checklist and the definition of daily care goals during multidisciplinary daily rounds and clinician prompts, can improve the in-hospital mortality of patients admitted to intensive care units (ICUs). Our secondary objectives are to assess the effects of the study intervention on specific processes of care, clinical outcomes, and the safety culture and to determine which factors (the processes of care and/or safety culture) mediate the effect of the study intervention on mortality.
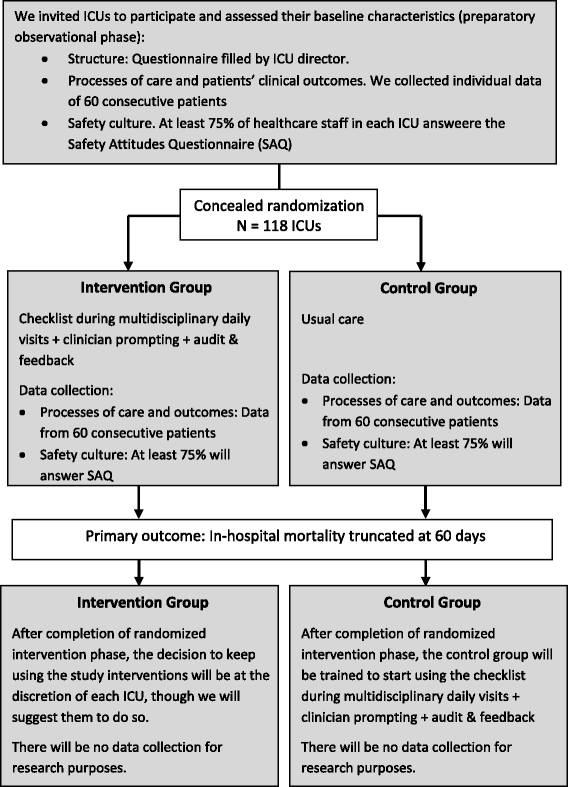
Methods/design: This is a cluster randomized trial involving 118 ICUs in Brazil conducted in two phases. In the observational preparatory phase, we collect baseline data on processes of care and clinical outcomes from 60 consecutive patients with lengths of ICU stay longer than 48 h and apply the Safety Attitudes Questionnaire (SAQ) to 75% or more of the health care staff in each ICU. In the randomized phase, we assign ICUs to the experimental or control arm and repeat data collection. Experimental arm ICUs receive the multifaceted quality improvement intervention, including a checklist and definition of daily care goals during daily multidisciplinary rounds, clinician prompting, and feedback on rates of adherence to selected care processes. Control arm ICUs maintain usual care. The primary outcome is in-hospital mortality, truncated at 60 days. Secondary outcomes include the rates of adherence to appropriate care processes, rates of other clinical outcomes, and scores on the SAQ domains. Analysis follows the intention-to-treat principle, and the primary outcome is analyzed using mixed effects logistic regression.
Discussion: This is a large scale, pragmatic cluster-randomized trial evaluating whether a multifaceted quality improvement intervention, including checklists applied during the multidisciplinary daily rounds and clinician prompting, can improve the adoption of proven therapies and decrease the mortality of critically ill patients. If this study finds that the intervention reduces mortality, it may be widely adopted in intensive care units, even those in limited-resource settings.
Trial registration: ClinicalTrials.gov NCT01785966.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical