Efficacy and safety of nicoboxil/nonivamide ointment for the treatment of acute pain in the low back - A randomized, controlled trial
- PMID: 25929250
- PMCID: PMC5029595
- DOI: 10.1002/ejp.719
Efficacy and safety of nicoboxil/nonivamide ointment for the treatment of acute pain in the low back - A randomized, controlled trial
Abstract
Background: Until now, nonivamide/nicoboxil ointment has not been tested in a randomized trial for the treatment of acute non-specific low back pain.
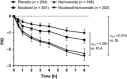
Methods: This phase III randomized, double-blind, active- and placebo-controlled, multi-centre trial investigated efficacy, safety and tolerability of topical nicoboxil 2.5%/nonivamide 0.4% for treatment of acute non-specific low back pain [primary endpoint: pain intensity (PI) difference between pre-dose baseline and 8 h after the first application].
Results: Patients (n = 805), 18-74 years of age were treated for up to 4 days with nicoboxil 2.5%/nonivamide 0.4%, nicoboxil 2.5%, nonivamide 0.4% or placebo ointment. Pre-dose baseline pain intensity (6.6 on a 0- to 10-point numerical rating scale) was reduced by 1.049 points with placebo, by 1.428 points with nicoboxil, by 2.252 points with nonivamide and by 2.410 points with nicoboxil/nonivamide after 8 h (p < 0.0001 for nicoboxil/nonivamide vs. placebo, nicoboxil; p = 0.4171 for nicoboxil/nonivamide vs. nonivamide). At the end of treatment, the combination provided more pronounced PI reduction (3.540 points) compared with nicoboxil (2.371, p < 0.0001), nonivamide (3.074, p = 0.0259) and placebo (1.884, p < 0.0001). Low back mobility scores on Day 1 were better for the combination compared with all other treatments (p < 0.044); on Day 2-4, scores were better than for placebo and nicoboxil (p < 0.003). Patients assessed efficacy of the combination as greater than of the comparators (p ≤ 0.0129). All treatments were tolerated well. No treatment-related serious adverse events were reported.
Conclusion: Nicoboxil/nonivamide ointment is an effective, well-tolerated medication for the treatment of acute non-specific low back pain.
Trial registration: ClinicalTrials.gov NCT01708915.
© 2015 The Authors. European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation - EFIC®
Figures

Similar articles
-
Nicoboxil/nonivamide cream effectively and safely reduces acute nonspecific low back pain - a randomized, placebo-controlled trial.J Pain Res. 2016 Dec 14;9:1221-1230. doi: 10.2147/JPR.S118329. eCollection 2016. J Pain Res. 2016. PMID: 28008281 Free PMC article.
-
Improved dorsal random-pattern skin flap survival in rats with a topically applied combination of nonivamide and nicoboxil.Plast Reconstr Surg. 2003 Mar;111(3):1207-11. doi: 10.1097/01.PRS.0000047404.28025.C5. Plast Reconstr Surg. 2003. PMID: 12621192
-
No Influence of Nonivamide-nicoboxil on the Peak Power Output in Competitive Sportsmen.Int J Sports Med. 2021 Nov;42(12):1092-1097. doi: 10.1055/a-1403-2701. Epub 2021 Apr 15. Int J Sports Med. 2021. PMID: 33860476 Free PMC article.
-
A comparison of pretreatment with a topical combination of nonivamide and nicoboxil and surgical delay in a random pattern skin flap model.J Plast Reconstr Aesthet Surg. 2009 Jul;62(7):914-9. doi: 10.1016/j.bjps.2007.11.054. Epub 2008 May 5. J Plast Reconstr Aesthet Surg. 2009. PMID: 18456588
-
Evaluation of changes in the haemoglobin of skin and muscle tissue of the calf, as induced by topical application of a nonivamide/nicoboxil cream.Can J Physiol Pharmacol. 2014 Feb;92(2):149-54. doi: 10.1139/cjpp-2013-0158. Epub 2013 Oct 21. Can J Physiol Pharmacol. 2014. PMID: 24502638
Cited by
-
Nicoboxil/nonivamide cream effectively and safely reduces acute nonspecific low back pain - a randomized, placebo-controlled trial.J Pain Res. 2016 Dec 14;9:1221-1230. doi: 10.2147/JPR.S118329. eCollection 2016. J Pain Res. 2016. PMID: 28008281 Free PMC article.
-
Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases.Molecules. 2016 Jul 23;21(8):966. doi: 10.3390/molecules21080966. Molecules. 2016. PMID: 27455231 Free PMC article. Review.
-
Author's Response to: 'Letter to the Editor Regarding Efficacy and Safety of Diclofenac and Capsaicin Gel in Patients with Acute Back/Neck Pain: A Multicenter Randomized Controlled Study'.Pain Ther. 2020 Dec;9(2):823-824. doi: 10.1007/s40122-020-00182-4. Epub 2020 Jul 9. Pain Ther. 2020. PMID: 32648204 Free PMC article. No abstract available.
-
Effectiveness and safety of tendon-bone-setting in postpartum women with sacroiliac joint pain: A retrospective multicenter study.Medicine (Baltimore). 2024 Jan 19;103(3):e36858. doi: 10.1097/MD.0000000000036858. Medicine (Baltimore). 2024. PMID: 38241584 Free PMC article.
-
Enhancing capillary blood collection: The influence of nicotinic acid and nonivamide.J Clin Lab Anal. 2017 Nov;31(6):e22142. doi: 10.1002/jcla.22142. Epub 2017 Jan 19. J Clin Lab Anal. 2017. PMID: 28102549 Free PMC article.
References
-
- Babej‐Doelle, R. , Freytag, S. , Eckmeyer, J. , Zerle, G. , Schinzel, S. , Schmieder, G. , Stankov, G. (1994). Parenteral dipyrone versus diclofenac and placebo in patients with acute lumbago or sciatic pain: Randomized observer‐blind multicenter study. Int J Clin Pharmacol Ther 32, 204–209. - PubMed
-
- Bley, K. (2013). Effects of topical capsaicin on cutaneous innervation: Implications for pain management. Open Pain J 6, 81–94.
-
- Buynak, R. , Shapiro, D.Y. , Okamoto, A. , Van Hove, I. , Rauschkolb, C. , Steup, A. , Lange, B. , Lange, C. , Etropolski, M. (2010). Efficacy and safety of tapentadol extended release for the management of chronic low back pain: Results of a prospective, randomized, double‐blind, placebo‐ and active‐controlled Phase III study. Expert Opin Pharmacother 11, 1787–1804. - PubMed
-
- Casser, H.‐R. (2012). Definition, Epidemiologie und soziooekonomische Bedeutung des nichtspezifischen Kreuzschmerz. Orthopaede 41, 311–312. - PubMed
-
- Chitil, W. , Ortner, H. (1959). Clinical experiences with Finalgon ointment in a total of 1040 cases. Prakt Arzt 13, 201–207.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

