Impaired development of left anterior heart field by ectopic retinoic acid causes transposition of the great arteries
- PMID: 25929268
- PMCID: PMC4599416
- DOI: 10.1161/JAHA.115.001889
Impaired development of left anterior heart field by ectopic retinoic acid causes transposition of the great arteries
Abstract
Background: Transposition of the great arteries is one of the most commonly diagnosed conotruncal heart defects at birth, but its etiology is largely unknown. The anterior heart field (AHF) that resides in the anterior pharyngeal arches contributes to conotruncal development, during which heart progenitors that originated from the left and right AHF migrate to form distinct conotruncal regions. The aim of this study is to identify abnormal AHF development that causes the morphology of transposition of the great arteries.
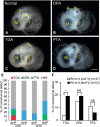
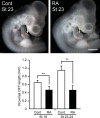
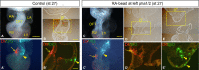
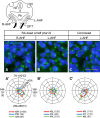
Methods and results: We placed a retinoic acid-soaked bead on the left or the right or on both sides of the AHF of stage 12 to 14 chick embryos and examined the conotruncal heart defect at stage 34. Transposition of the great arteries was diagnosed at high incidence in embryos for which a retinoic acid-soaked bead had been placed in the left AHF at stage 12. Fluorescent dye tracing showed that AHF exposed to retinoic acid failed to contribute to conotruncus development. FGF8 and Isl1 expression were downregulated in retinoic acid-exposed AHF, and differentiation and expansion of cardiomyocytes were suppressed in cultured AHF in medium supplemented with retinoic acid.
Conclusions: The left AHF at the early looped heart stage, corresponding to Carnegie stages 10 to 11 (28 to 29 days after fertilization) in human embryos, is the region of the impediment that causes the morphology of transposition of the great arteries.
Keywords: Isl1; congenital; heart defects; morphogenesis; transposition of great vessel.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures

Similar articles
-
Mechanism responsible for D-transposition of the great arteries: Is this part of the spectrum of right isomerism?Congenit Anom (Kyoto). 2016 Sep;56(5):196-202. doi: 10.1111/cga.12176. Congenit Anom (Kyoto). 2016. PMID: 27329052 Review.
-
Morphological observations on the pathogenetic process of transposition of the great arteries induced by retinoic acid in mice.Circulation. 1995 May 1;91(9):2478-86. doi: 10.1161/01.cir.91.9.2478. Circulation. 1995. PMID: 7729035
-
A multiple retinoic acid antagonist induces conotruncal anomalies, including transposition of the great arteries, in mice.Cardiovasc Pathol. 2006 Jul-Aug;15(4):194-202. doi: 10.1016/j.carpath.2006.04.004. Cardiovasc Pathol. 2006. PMID: 16844550
-
Myocardial progenitors in the pharyngeal regions migrate to distinct conotruncal regions.Dev Dyn. 2012 Feb;241(2):284-93. doi: 10.1002/dvdy.23714. Epub 2011 Dec 19. Dev Dyn. 2012. PMID: 22184055
-
Second lineage of heart forming region provides new understanding of conotruncal heart defects.Congenit Anom (Kyoto). 2010 Mar;50(1):8-14. doi: 10.1111/j.1741-4520.2009.00267.x. Epub 2009 Dec 24. Congenit Anom (Kyoto). 2010. PMID: 20050864 Review.
Cited by
-
Role of carotenoids and retinoids during heart development.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Nov;1865(11):158636. doi: 10.1016/j.bbalip.2020.158636. Epub 2020 Jan 22. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31978553 Free PMC article. Review.
-
Prenatal retinoic acid exposure reveals candidate genes for craniofacial disorders.Sci Rep. 2018 Nov 30;8(1):17492. doi: 10.1038/s41598-018-35681-0. Sci Rep. 2018. PMID: 30504818 Free PMC article.
-
Mutations in Hnrnpa1 cause congenital heart defects.JCI Insight. 2018 Jan 25;3(2):e98555. doi: 10.1172/jci.insight.98555. eCollection 2018 Jan 25. JCI Insight. 2018. PMID: 29367466 Free PMC article.
-
An essential role for the nuclear protein Akirin2 in mouse limb interdigital tissue regression.Sci Rep. 2018 Aug 16;8(1):12240. doi: 10.1038/s41598-018-30801-2. Sci Rep. 2018. PMID: 30116001 Free PMC article.
References
-
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. - PubMed
-
- Ferencz C, Brenner JI, Loffredo C, Kappetein AP, Wilson PD. Transposition of the great arteries: etiologic distinctions of outflow tract defects in a case control study of risk factors. In: Clark EB, Markwald RR, Takao A, editors. Developmental Mechanisms of Heart Disease. New York, NY: Futura Publishing; 1995. pp. 639–653.
-
- Bostrom MP, Hutchins GM. Arrested rotation of the outflow tract may explain double-outlet right ventricle. Circulation. 1988;77:1258–1265. - PubMed
-
- Lomonico MP, Bostrom MP, Moore GW, Hutchins GM. Arrested rotation of the outflow tract may explain tetralogy of Fallot and transposition of the great arteries. Pediatr Pathol. 1988;8:267–281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

