Probiotics for standard triple Helicobacter pylori eradication: a randomized, double-blind, placebo-controlled trial
- PMID: 25929897
- PMCID: PMC4603068
- DOI: 10.1097/MD.0000000000000685
Probiotics for standard triple Helicobacter pylori eradication: a randomized, double-blind, placebo-controlled trial
Abstract
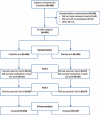
The primary objective in the study is determination of efficacy of probiotic preparation as a supportive therapy in eradication of Helicobacter pylori.The study was multicenter, prospective, randomized, placebo controlled, and double-blind. The subjects first filled out a specially designed questionnaire to assess the severity of the 10 symptoms, which can be related to eradication therapy to be monitored during the trial. Each subject then received 28 capsules of probiotic preparation or matching placebo capsules, which they were supposed to take over the following 14 days, twice a day, at least 2 hours prior to or after the antibiotic therapy administration.A total of 804 patients were enrolled in the trial, of which 650 (80.85%) were included in the analysis. The results show a significantly larger share of cured subjects in the probiotic arm versus the placebo arm (87.38% vs 72.55%; P < 0.001). Additionally, presence and intensity of epigastric pain, bloating, flatulence, taste disturbance, loss of appetite, nausea, vomiting, heartburn, rash, and diarrhea were monitored over the study period. At 15 days postinclusion, probiotic treatment was found superior to placebo in 7 of 10 mentioned symptoms. Average intensity for symptoms potentially related to antibiotic therapy was significantly higher in the placebo group, 0.76 vs 0.55 (P < 0.001).Adding probiotics to the standard triple therapy for H pylori eradication significantly contributes to treatment efficacy and distinctly decreases the adverse effects of therapy and the symptoms of the underlying disease.
Figures
References
-
- Metchnikoff E. The prolongation of life. Optimistic studies. 1908; New York: Putman's Sons, 161–183.
-
- Marteau PR, de Vrese M, Cellier CJ, et al. Protection from gastrointestinal diseases with the use of probiotics. Am J Clin Nutr 2001; 73:430S–436S. - PubMed
-
- Gotz V, Romankiewicz JA, Moss J, et al. Prophylaxis against ampicillin-associated diarrhea with a lactobacillus preparation. Am J Hosp Pharm 1979; 36:754–757. - PubMed
-
- Borgia M, Sepe N, Brancato V, et al. A controlled clinical study on Streptococcus faecium preparation for the prevention of side reactions during long-term antibiotic treatments. Curr Ther Res 1982; 31:265–271.
-
- Clements ML, Levine MM, Ristiano PA, et al. Exogenous lactobacilli fed to man. Their fate and ability to prevent diarrheal disease. Prog Food Nutr Sci 1983; 7:29–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical