Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension
- PMID: 25930027
- PMCID: PMC4816410
- DOI: 10.1152/japplphysiol.00283.2015
Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension
Abstract
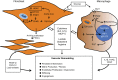
Pulmonary hypertension (PH) is a complex, multifactorial syndrome that remains poorly understood despite decades of research. PH is characterized by profound pulmonary artery (PA) remodeling that includes significant fibro-proliferative and inflammatory changes of the PA adventitia. In line with the emerging concept that PH shares key features with cancer, recent work centers on the idea that PH results from a multistep process driven by reprogramming of gene-expression patterns that govern changes in cell metabolism, inflammation, and proliferation. Data demonstrate that in addition to PA endothelial cells and smooth muscle cells, adventitial fibroblasts from animals with experimental hypoxic PH and from humans with PH (hereafter, termed PH-Fibs) exhibit proinflammatory activation, increased proliferation, and apoptosis resistance, all in the context of metabolic reprogramming to aerobic glycolysis. PH-Fibs can also recruit, retain, and activate naïve macrophages (Mϕ) toward a proinflammatory/proremodeling phenotype through secretion of chemokines, cytokines, and glycolytic metabolites, among which IL-6 and lactate play key roles. Furthermore, these fibroblast-activated Mϕ (hereafter, termed FAMϕ) exhibit aerobic glycolysis together with high expression of arginase 1, Vegfa, and I1lb, all of which require hypoxia-inducible factor 1α and STAT3 signaling. Strikingly, in situ, the adventitial Mϕ phenotype in the remodeled PA closely resembles the Mϕ phenotype induced by fibroblasts in vitro (FAMϕ), suggesting that FAMϕ crosstalk involving metabolic and inflammatory signals is a critical, pathogenetic component of vascular remodeling. This review discusses metabolic and inflammatory changes in fibroblasts and Mϕ in PH with the goal of raising ideas about new interventions to abrogate remodeling in hypoxic forms of PH.
Keywords: HIF; IL-6; fibroblast; glycolysis; macrophage.
Copyright © 2015 the American Physiological Society.
Figures
References
-
- Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 93: 391–398, 1980. - PubMed
-
- Anwar A, Li M, Frid MG, Kumar B, Gerasimovskaya EV, Riddle SR, McKeon BA, Thukaram R, Meyrick BO, Fini MA, Stenmark KR. Osteopontin is an endogenous modulator of the constitutively activated phenotype of pulmonary adventitial fibroblasts in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 303: L1–L11, 2012. - PMC - PubMed
-
- Baglole CJ, Ray DM, Bernstein SH, Feldon SE, Smith TJ, Sime PJ, Phipps RP. More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunol Invest 35: 297–325, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

