Epigenetic regulation of germ cells-remember or forget?
- PMID: 25930104
- PMCID: PMC4470759
- DOI: 10.1016/j.gde.2015.04.003
Epigenetic regulation of germ cells-remember or forget?
Abstract
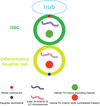
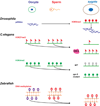
Unlike somatic cells, germ cells retain the potential to reproduce an entire new organism upon fertilization. In order to accomplish the process of fertilization, germ cells undergo an extreme cellular differentiation process known as gametogenesis in order to produce morphologically and functionally distinct oocyte and sperm. In addition to changes in genetic content changes from diploid to haploid, epigenetic mechanisms that modify chromatin state without altering primary DNA sequences have profound influence on germ cell differentiation and moreover, the transgenerational effect. In this review, we will go over the most recent discoveries on epigenetic regulations in germline differentiation and transgenerational inheritance across different metazoan species.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Protecting and Diversifying the Germline.Genetics. 2018 Feb;208(2):435-471. doi: 10.1534/genetics.117.300208. Genetics. 2018. PMID: 29378808 Free PMC article. Review.
-
Epigenetic regulation of germ cell differentiation.Curr Opin Cell Biol. 2010 Dec;22(6):737-43. doi: 10.1016/j.ceb.2010.09.004. Epub 2010 Oct 13. Curr Opin Cell Biol. 2010. PMID: 20951019 Free PMC article. Review.
-
Of stem cells and gametes: similarities and differences.Curr Med Chem. 2008;15(13):1249-56. doi: 10.2174/092986708784534992. Curr Med Chem. 2008. PMID: 18537604 Review.
-
Specifying and protecting germ cell fate.Nat Rev Mol Cell Biol. 2015 Jul;16(7):406-16. doi: 10.1038/nrm4009. Nat Rev Mol Cell Biol. 2015. PMID: 26122616 Free PMC article. Review.
-
Epigenetic transgenerational inheritance, gametogenesis and germline development†.Biol Reprod. 2021 Sep 14;105(3):570-592. doi: 10.1093/biolre/ioab085. Biol Reprod. 2021. PMID: 33929020 Free PMC article. Review.
Cited by
-
Profiling the male germline genome to unravel its reproductive potential.Fertil Steril. 2023 Feb;119(2):196-206. doi: 10.1016/j.fertnstert.2022.11.006. Epub 2022 Nov 12. Fertil Steril. 2023. PMID: 36379263 Free PMC article.
-
Genome-wide 5-hydroxymethylcytosine patterns in human spermatogenesis are associated with semen quality.Oncotarget. 2017 Jun 1;8(51):88294-88307. doi: 10.18632/oncotarget.18331. eCollection 2017 Oct 24. Oncotarget. 2017. PMID: 29179435 Free PMC article.
-
The protamines of the noble false widow spider Steatoda nobilis provide an example of liquid-liquid phase separation chromatin transitions during spermiogenesis.bioRxiv [Preprint]. 2024 Jun 5:2024.06.04.597381. doi: 10.1101/2024.06.04.597381. bioRxiv. 2024. Update in: Development. 2024 Nov 15;151(22):dev203134. doi: 10.1242/dev.203134. PMID: 38895387 Free PMC article. Updated. Preprint.
-
dRTEL1 is essential for the maintenance of Drosophila male germline stem cells.PLoS Genet. 2021 Oct 13;17(10):e1009834. doi: 10.1371/journal.pgen.1009834. eCollection 2021 Oct. PLoS Genet. 2021. PMID: 34644293 Free PMC article.
-
Enhancer of polycomb coordinates multiple signaling pathways to promote both cyst and germline stem cell differentiation in the Drosophila adult testis.PLoS Genet. 2017 Feb 14;13(2):e1006571. doi: 10.1371/journal.pgen.1006571. eCollection 2017 Feb. PLoS Genet. 2017. PMID: 28196077 Free PMC article.
References
-
- Richards EJ, Elgin SC. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell. 2002;108(4):489–500. - PubMed
-
- Lyko F, Ramsahoye BH, Jaenisch R. DNA methylation in Drosophila melanogaster. Nature. 2000;408(6812):538–540. - PubMed
-
- Lyko F, et al. The putative Drosophila methyltransferase gene dDnmt2 is contained in a transposon-like element and is expressed specifically in ovaries. Mech Dev. 2000;95(1–2):215–217. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

