A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients
- PMID: 25930144
- PMCID: PMC5034803
- DOI: 10.1111/1755-5922.12128
A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients
Abstract
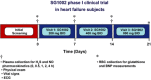
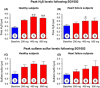
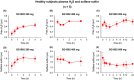
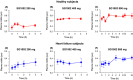
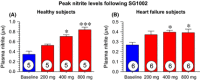
Recent studies demonstrate robust molecular cross talk and signaling between hydrogen sulfide (H2 S) and nitric oxide (NO). Heart failure (HF) patients are deficient in both H2 S and NO, two molecules that are critical for cardiovascular homeostasis. A phase I clinical trial of a novel H2 S prodrug (SG1002) was designed to assess safety and changes in H2 S and NO bioavailability in healthy and HF subjects. Healthy subjects (n = 7) and heart failure patients (n = 8) received oral SG1002 treatment in escalating dosages of 200, 400, and 800 mg twice daily for 7 days for each dose. Safety and tolerability were assessed by physical examination, vital signs, and ECG analysis. Plasma samples were collected during a 24-h period each week for H2 S and NO analysis. BNP and glutathione levels were analyzed as markers of cardiac health and redox status. Administration of SG1002 resulted in increased H2 S levels in healthy subjects. We also observed increased H2 S levels in HF subjects following 400 mg SG1002. Nitrite, a metabolite of NO, was increased in both healthy and HF patients receiving 400 mg and 800 mg SG1002. HF subjects treated with SG1002 displayed stable drug levels over the course of the trial. SG1002 was safe and well tolerated at all doses in both healthy and HF subjects. These data suggest that SG1002 increases blood H2 S levels and circulating NO bioavailability. The finding that SG1002 attenuates increases in BNP in HF patients suggests that this novel agent warrants further study in a larger clinical study.
Trial registration: ClinicalTrials.gov NCT01989208.
Keywords: Nitrite; Oxidative stress; Phase 1 clinical trial; Sulfide.
© 2015 SulfaGENIX Inc. Cardiovascular Therapeutics published by John Wiley & Sons Ltd.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

