Arginase: an old enzyme with new tricks
- PMID: 25930708
- PMCID: PMC4461463
- DOI: 10.1016/j.tips.2015.03.006
Arginase: an old enzyme with new tricks
Abstract
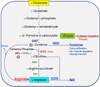
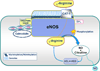
Arginase has roots in early life-forms. It converts L-arginine to urea and ornithine. The former provides protection against NH3; the latter serves to stimulate cell growth and other physiological functions. Excessive arginase activity in mammals has been associated with cardiovascular and nervous system dysfunction and disease. Two relevant aspects of this elevated activity may be involved in these disease states. First, excessive arginase activity reduces the supply of L-arginine needed by nitric oxide (NO) synthase to produce NO. Second, excessive production of ornithine leads to vascular structural problems and neural toxicity. Recent research has identified inflammatory agents and reactive oxygen species (ROS) as drivers of this pathologic elevation of arginase activity and expression. We review the involvement of arginase in cardiovascular and nervous system dysfunction, and discuss potential therapeutic interventions targeting excess arginase.
Keywords: arginase; neurodegeneration; nitric oxide; oxidative stress; peroxynitrite; polyamine; superoxide; vascular dysfunction.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


References
-
- Dizikes GJ, et al. Isolation of human liver arginase cDNA and demonstration of nonhomology between the two human arginase genes. Biochem Biophys Res Commun. 1986;141:53–59. - PubMed
-
- Gotoh T, et al. Molecular cloning of cDNA for nonhepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett. 1996;395:119–122. - PubMed
-
- Vockley JG, et al. Cloning and characterization of the human type II arginase gene. Genomics. 1996;38:118–123. - PubMed
-
- Ash DE. Structure and function of arginases. J Nutr. 2004;134:2760S–2764S. discussion 2765S–2767S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

