Effects of luseogliflozin, a sodium-glucose co-transporter 2 inhibitor, on 24-h glucose variability assessed by continuous glucose monitoring in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, crossover study
- PMID: 25930989
- PMCID: PMC5032984
- DOI: 10.1111/dom.12481
Effects of luseogliflozin, a sodium-glucose co-transporter 2 inhibitor, on 24-h glucose variability assessed by continuous glucose monitoring in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, crossover study
Abstract
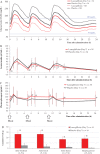
The aim of the present study was to determine the effects of luseogliflozin on 24-h glucose levels, assessed by continuous glucose monitoring, and on pharmacodynamic variables measured throughout the day. In this double-blind, placebo-controlled, crossover study, 37 patients with type 2 diabetes mellitus inadequately controlled with diet and exercise were randomized into two groups. Patients in each group first received luseogliflozin then placebo for 7 days each, or vice versa. After 7 days of treatment, the mean 24-h glucose level was significantly lower with luseogliflozin than with placebo [mean (95% confidence interval) 145.9 (134.4-157.5) mg/dl vs 168.5 (156.9-180.0) mg/dl; p < 0.001]. The proportion of time spent with glucose levels ≥70 to ≤180 mg/dl was significantly greater with luseogliflozin than with placebo [median (interquartile range) 83.2 (67.7-96.5)% vs 71.9 (46.9-83.3)%; p < 0.001] without inducing hypoglycaemia. The decrease in glucose levels was accompanied by reductions in serum insulin levels throughout the day.
Keywords: 24-h glucose variability; SGLT2 inhibitor; continuous glucose monitoring; luseogliflozin; type 2 diabetes.
© 2015 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
References
-
- Markham A, Elkinson S. Luseogliflozin: first global approval. Drugs 2014; 74: 945–950. - PubMed
-
- Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled, phase 3 study. Curr Med Res Opin 2014; 30: 1245–1255. - PubMed
-
- Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 2012; 8: 495–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

