Genistein increases estrogen receptor beta expression in prostate cancer via reducing its promoter methylation
- PMID: 25931004
- PMCID: PMC4501888
- DOI: 10.1016/j.jsbmb.2015.04.018
Genistein increases estrogen receptor beta expression in prostate cancer via reducing its promoter methylation
Abstract
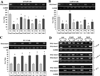
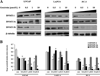
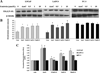
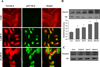
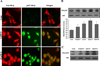
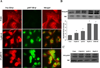
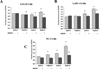
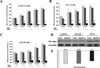
Genistein has protective effects against prostate cancer (PCa) but whether this protection involves an estrogen receptor (ER) β dependent mechanism has yet to be elucidated. ER-β has a tumor suppressor role in PCa and its levels decline with cancer progression which was linked to ER-β promoter hypermethylation. Genistein has been suggested to have demethylating activities in cancer. However, the ability of genistein to reverse ER-β promoter hypermethylation in PCa has not been studied. In addition, there are great discrepancies among studies that examined the effect of genistein on ER-β gene expression. Therefore, we sought to explore effects of genistein on ER-β promoter methylation as a mechanism of modulating ER-β expression using three PCa cell lines, LNCaP, LAPC-4 and PC-3. We also examined the role of ER-β in mediating the preventive action of genistein. Our data demonstrated that genistein at physiological ranges (0.5-10 μmol/L) reduced ER-β promoter methylation significantly with corresponding dose-dependent increases in ER-β expression in LNCaP and LAPC-4 but not in PC-3 cells, which could be attributed to the low basal levels of ER-β promoter methylation in PC-3 cell line. Genistein induced phosphorylation, nuclear translocation and transcriptional activity of ER-β in all three PCa cell lines. Inhibitory effects of genistein on LAPC-4 and PC-3 cell proliferation were diminished using a specific ER-β antagonist. In conclusion, genistein and ER-β act together to prevent PCa cell proliferation; genistein increases ER-β levels via reducing its promoter methylation and ER-β, in turn, mediates the preventive action of genistein.
Keywords: Estrogen receptor β; Genistein; Promoter methylation; Prostate cancer.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
DNA methylation paradigm shift: 15-lipoxygenase-1 upregulation in prostatic intraepithelial neoplasia and prostate cancer by atypical promoter hypermethylation.Prostaglandins Other Lipid Mediat. 2007 Jan;82(1-4):185-97. doi: 10.1016/j.prostaglandins.2006.05.015. Epub 2006 Jul 11. Prostaglandins Other Lipid Mediat. 2007. PMID: 17164146
-
Comparative effects of soy phytoestrogens and 17β-estradiol on DNA methylation of a panel of 24 genes in prostate cancer cell lines.Nutr Cancer. 2014;66(3):474-82. doi: 10.1080/01635581.2014.884236. Epub 2014 Mar 18. Nutr Cancer. 2014. PMID: 24641702
-
DNA methylation and soy phytoestrogens: quantitative study in DU-145 and PC-3 human prostate cancer cell lines.Epigenomics. 2011 Dec;3(6):795-803. doi: 10.2217/epi.11.103. Epigenomics. 2011. PMID: 22126297
-
Estrogens and antiestrogens as etiological factors and therapeutics for prostate cancer.Ann N Y Acad Sci. 2006 Nov;1089:177-93. doi: 10.1196/annals.1386.005. Ann N Y Acad Sci. 2006. PMID: 17261766 Review.
-
Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates.J Cell Biochem. 2004 Feb 15;91(3):491-503. doi: 10.1002/jcb.10759. J Cell Biochem. 2004. PMID: 14755680 Review.
Cited by
-
Dual role of Endoplasmic Reticulum Stress-Mediated Unfolded Protein Response Signaling Pathway in Carcinogenesis.Int J Mol Sci. 2019 Sep 5;20(18):4354. doi: 10.3390/ijms20184354. Int J Mol Sci. 2019. PMID: 31491919 Free PMC article. Review.
-
A Mini-Review of Flavone Isomers Apigenin and Genistein in Prostate Cancer Treatment.Front Pharmacol. 2022 Mar 11;13:851589. doi: 10.3389/fphar.2022.851589. eCollection 2022. Front Pharmacol. 2022. PMID: 35359832 Free PMC article. Review.
-
Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer.Int J Mol Sci. 2017 Jun 1;18(6):1178. doi: 10.3390/ijms18061178. Int J Mol Sci. 2017. PMID: 28587155 Free PMC article. Review.
-
Involvement of estrogen receptor β in androgen receptor-induced growth inhibition in prostate cancer PC-3 cells.Oncol Lett. 2017 Sep;14(3):2796-2802. doi: 10.3892/ol.2017.6544. Epub 2017 Jul 8. Oncol Lett. 2017. PMID: 28928821 Free PMC article.
-
Targeting DNA Methyltranferases in Urological Tumors.Front Pharmacol. 2018 Apr 13;9:366. doi: 10.3389/fphar.2018.00366. eCollection 2018. Front Pharmacol. 2018. PMID: 29706891 Free PMC article. Review.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Siegel R, Desantis C, Virgo K, Stein K, Mariotto A, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2012;2012;62:220–241. - PubMed
-
- Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. - PubMed
-
- Morton MS, Chan PS, Cheng C, Blacklock N, Matos-Ferreira A, et al. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostate. 1997;32:122–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

