Analysis of Human Dopamine D3 Receptor Quaternary Structure
- PMID: 25931118
- PMCID: PMC4463457
- DOI: 10.1074/jbc.M114.630681
Analysis of Human Dopamine D3 Receptor Quaternary Structure
Abstract
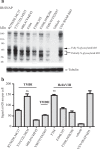
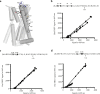
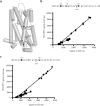
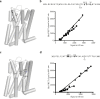
The dopamine D3 receptor is a class A, rhodopsin-like G protein-coupled receptor that can form dimers and/or higher order oligomers. However, the molecular basis for production of these complexes is not well defined. Using combinations of molecular modeling, site-directed mutagenesis, and homogenous time-resolved FRET, the interfaces that allow dopamine D3 receptor monomers to interact were defined and used to describe likely quaternary arrangements of the receptor. These were then compared with published crystal structures of dimeric β1-adrenoreceptor, μ-opioid, and CXCR4 receptors. The data indicate important contributions of residues from within each of transmembrane domains I, II, IV, V, VI, and VII as well as the intracellular helix VIII in the formation of D3-D3 receptor interfaces within homo-oligomers and are consistent with the D3 receptor adopting a β1-adrenoreceptor-like quaternary arrangement. Specifically, results suggest that D3 protomers can interact with each other via at least two distinct interfaces: the first one comprising residues from transmembrane domains I and II along with those from helix VIII and a second one involving transmembrane domains IV and V. Moreover, rather than existing only as distinct dimeric species, the results are consistent with the D3 receptor also assuming a quaternary structure in which two transmembrane domain I-II-helix VIII dimers interact to form a "rhombic" tetramer via an interface involving residues from transmembrane domains VI and VII. In addition, the results also provide insights into the potential contribution of molecules of cholesterol to the overall organization and potential stability of the D3 receptor and possibly other GPCR quaternary structures.
Keywords: G protein-coupled receptor, dopamine receptor, homodimer, tetramer, GPCR quaternary structure, molecular modeling, fluorescence resonance energy transfer.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures









References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

