MicroRNA-193a-3p Reduces Intestinal Inflammation in Response to Microbiota via Down-regulation of Colonic PepT1
- PMID: 25931122
- PMCID: PMC4481212
- DOI: 10.1074/jbc.M115.659318
MicroRNA-193a-3p Reduces Intestinal Inflammation in Response to Microbiota via Down-regulation of Colonic PepT1
Abstract
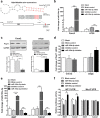
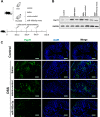
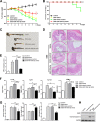
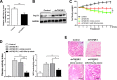
Intestinal inflammation is characterized by epithelial disruption, leading to the loss of barrier function, recruitment of immune cells, and host immune responses to gut microbiota. PepT1, a di/tripeptide transporter that uptakes bacterial products, is up-regulated in inflamed colon tissue, which implies its role in bacterium-associated intestinal inflammation. Although microRNA (miRNA)-mediated gene regulation has been found to be involved in various processes of inflammatory bowel disease (IBD), the biological function of miRNAs in the pathogenesis of IBD remains to be explored. In this study we detected miRNA expression patterns in colon tissues during colitis and investigated the mechanism underlying the regulation of colonic PepT1 by miRNAs. We observed an inverse correlation between PepT1 and miR-193a-3p in inflamed colon tissues with active ulcerative colitis, and we further demonstrated that miR-193a-3p reduced PepT1 expression and activity as a target gene and subsequently suppressed the NF-κB pathway. Intracolonic delivery of miR-193a-3p significantly ameliorated dextran sodium sulfate-induced colitis, whereas the overexpression of colonic PepT1 via PepT1 3'-untranslated region mutant lentivirus vector abolished the anti-inflammatory effect of miR-193a-3p. Furthermore, antibiotic treatment eliminated the difference in the dextran sodium sulfate-induced inflammation between the presence and absence of miR-193a-3p. These findings suggest that miR-193a-3p regulation of PepT1 mediates the uptake of bacterial products and is a potent mechanism during the colonic inflammation process. Overall, we believe miR-193a-3p may be a potent regulator of colonic PepT1 for maintaining intestinal homeostasis.
Keywords: gene regulation; inflammatory bowel disease (IBD); microRNA (miRNA); mucosal immunology; peptide transport.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Jostins L., Ripke S., Weersma R. K., Duerr R. H., McGovern D. P., Hui K. Y., Lee J. C., Schumm L. P., Sharma Y., Anderson C. A., Essers J., Mitrovic M., Ning K., Cleynen I., Theatre E., Spain S. L., Raychaudhuri S., Goyette P., Wei Z., Abraham C., Achkar J. P., Ahmad T., Amininejad L., Ananthakrishnan A. N., Andersen V., Andrews J. M.., Baidoo L., Balschun T., Bampton P. A., Bitton A., Boucher G., Brand S., Büning C., Cohain A., Cichon S., D'Amato M., De Jong D., Devaney K. L., Dubinsky M., Edwards C., Ellinghaus D., Ferguson L. R., Franchimont D., Fransen K., Gearry R., Georges M., Gieger C., Glas J., Haritunians T., Hart A., Hawkey C. (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 - PMC - PubMed
-
- Adibi S. A. (1997) The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology 113, 332–340 - PubMed
-
- Merlin D., Si-Tahar M., Sitaraman S. V., Eastburn K., Williams I., Liu X., Hediger M. A., Madara J. L. (2001) Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology 120, 1666–1679 - PubMed
-
- Adibi S. A. (2003) Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G779–G788 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

