Granulocyte/Macrophage Colony-stimulating Factor-dependent Dendritic Cells Restrain Lean Adipose Tissue Expansion
- PMID: 25931125
- PMCID: PMC4505532
- DOI: 10.1074/jbc.M115.645820
Granulocyte/Macrophage Colony-stimulating Factor-dependent Dendritic Cells Restrain Lean Adipose Tissue Expansion
Abstract
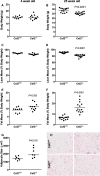
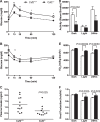
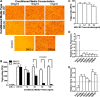
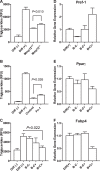
The physiological roles of macrophages and dendritic cells (DCs) in lean white adipose tissue homeostasis have received little attention. Because DCs are generated from bone marrow progenitors in the presence of granulocyte/macrophage colony-stimulating factor (GM-CSF), we used GM-CSF-deficient (Csf2(-/-)) mice fed a low fat diet to test the hypothesis that adipose tissue DCs regulate the development of adipose tissue. At 4 weeks of age, Csf2(-/-) mice had 75% fewer CD45(+)Cd11b(+)Cd11c(+)MHCII(+) F4/80(-) DCs in white adipose tissue than did wild-type controls. Furthermore, the Csf2(-/-) mice showed a 30% increase in whole body adiposity, which persisted to adulthood. Adipocytes from Csf2(-/-) mice were 50% larger by volume and contained higher levels of adipogenesis gene transcripts, indicating enhanced adipocyte differentiation. In contrast, adipogenesis/adipocyte lipid accumulation was inhibited when preadipocytes were co-cultured with CD45(+)Cd11b(+)Cd11c(+)MHCII(+)F4/80(-) DCs. Medium conditioned by DCs, but not by macrophages, also inhibited adipocyte lipid accumulation. Proteomic analysis revealed that matrix metalloproteinase 12 and fibronectin 1 were greatly enriched in the medium conditioned by DCs compared with that conditioned by macrophages. Silencing fibronectin or genetic deletion of matrix metalloproteinase 12 in DCs partially reversed the inhibition of adipocyte lipid accumulation. Our observations indicate that DCs residing in adipose tissue play a critical role in suppressing normal adipose tissue expansion.
Keywords: mice, GM-CSF, dendritic cells, adipose tissue.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Nishimura S., Manabe I., Nagasaki M., Hosoya Y., Yamashita H., Fujita H., Ohsugi M., Tobe K., Kadowaki T., Nagai R., Sugiura S. (2007) Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 56, 1517–1526 - PubMed
-
- Rosenson R. S., Brewer H. B., Jr., Chapman M. J., Fazio S., Hussain M. M., Kontush A., Krauss R. M., Otvos J. D., Remaley A. T., Schaefer E. J. (2011) HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 57, 392–410 - PubMed
-
- Morrison R. F., Farmer S. R. (2000) Hormonal signaling and transcriptional control of adipocyte differentiation. J. Nutr. 130, 3116S–3121S - PubMed
-
- O'Connell J., Lynch L., Hogan A., Cawood T. J., O'Shea D. (2011) Preadipocyte factor-1 is associated with metabolic profile in severe obesity. J. Clin. Endocrinol. Metab. 96, E680–E684 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL092969/HL/NHLBI NIH HHS/United States
- R01 HL097365/HL/NHLBI NIH HHS/United States
- R01 HL112625/HL/NHLBI NIH HHS/United States
- R01 DK093493/DK/NIDDK NIH HHS/United States
- R01 HL062887/HL/NHLBI NIH HHS/United States
- R01 HL108897/HL/NHLBI NIH HHS/United States
- HL097365/HL/NHLBI NIH HHS/United States
- HL112625/HL/NHLBI NIH HHS/United States
- HL108897/HL/NHLBI NIH HHS/United States
- P30 DK035816/DK/NIDDK NIH HHS/United States
- P01 HL092969/HL/NHLBI NIH HHS/United States
- HL062887/HL/NHLBI NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

