NEURODEVELOPMENT. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells
- PMID: 25931449
- PMCID: PMC4652794
- DOI: 10.1126/science.aaa3655
NEURODEVELOPMENT. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells
Erratum in
-
Erratum for the Research Article "Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells" by E. Buitrago-Delgado et al.Science. 2023 Aug 11;381(6658):eadk1062. doi: 10.1126/science.adk1062. Epub 2023 Aug 10. Science. 2023. PMID: 37561881 No abstract available.
Abstract
Neural crest cells, which are specific to vertebrates, arise in the ectoderm but can generate cell types that are typically categorized as mesodermal. This broad developmental potential persists past the time when most ectoderm-derived cells become lineage-restricted. The ability of neural crest to contribute mesodermal derivatives to the bauplan has raised questions about how this apparent gain in potential is achieved. Here, we describe shared molecular underpinnings of potency in neural crest and blastula cells. We show that in Xenopus, key neural crest regulatory factors are also expressed in blastula animal pole cells and promote pluripotency in both cell types. We suggest that neural crest cells may have evolved as a consequence of a subset of blastula cells retaining activity of the regulatory network underlying pluripotency.
Copyright © 2015, American Association for the Advancement of Science.
Figures




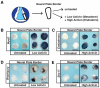
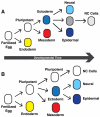
Comment in
-
DEVELOPMENTAL BIOLOGY. It's about time for neural crest.Science. 2015 Jun 19;348(6241):1316-7. doi: 10.1126/science.aab2719. Epub 2015 Apr 30. Science. 2015. PMID: 25931447 No abstract available.
References
-
- Furue M, Asashima M. In: Handbook of Stem Cells. 46. Lanza R, et al., editors. Vol. 1. Elsevier; Burlington, San Diego, London: 2004.
-
- Hall BK. The Neural Crest in Development and Evolution. 1 Springer; New York: 1999.
-
- Light W, Vernon AE, Lasorella A, Iavarone A, LaBonne C. Xenopus Id3 is required downstream of Myc for the formation of multipotent neural crest progenitor cells. Development. 2005;132:1831–1841. - PubMed
-
- Bellmeyer A, Krase J, Lindgren J, LaBonne C. The protooncogene c-myc is an essential regulator of neural crest formation in xenopus. Dev Cell. 2003;4:827–839. - PubMed
-
- Cartwright P, et al. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Mycdependent mechanism. Development. 2005;132:885–896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

