Bacterial division. Mechanical crack propagation drives millisecond daughter cell separation in Staphylococcus aureus
- PMID: 25931560
- PMCID: PMC4864021
- DOI: 10.1126/science.aaa1511
Bacterial division. Mechanical crack propagation drives millisecond daughter cell separation in Staphylococcus aureus
Abstract
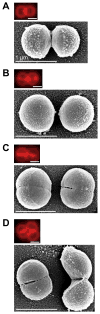
When Staphylococcus aureus undergoes cytokinesis, it builds a septum, generating two hemispherical daughters whose cell walls are only connected via a narrow peripheral ring. We found that resolution of this ring occurred within milliseconds ("popping"), without detectable changes in cell volume. The likelihood of popping depended on cell-wall stress, and the separating cells split open asymmetrically, leaving the daughters connected by a hinge. An elastostatic model of the wall indicated high circumferential stress in the peripheral ring before popping. Last, we observed small perforations in the peripheral ring that are likely initial points of mechanical failure. Thus, the ultrafast daughter cell separation in S. aureus appears to be driven by accumulation of stress in the peripheral ring and exhibits hallmarks of mechanical crack propagation.
Copyright © 2015, American Association for the Advancement of Science.
Figures


References
-
- Wu LJ, Errington J. Nucleoid occlusion and bacterial cell division. Nat Rev Microbiol. 2012;10:8–12. - PubMed
-
- Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. - PubMed
-
- Pinho MG, Kjos M, Veening JW. How to get (a)round: mechanisms controlling growth and division of coccoid bacteria. Nat Rev Microbiol. 2013;11:601–614. - PubMed
Publication types
MeSH terms
Grants and funding
- U54-GM072970/GM/NIGMS NIH HHS/United States
- P50 GM107615/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32-GM007276/GM/NIGMS NIH HHS/United States
- R37 AI036929/AI/NIAID NIH HHS/United States
- P50-GM107615/GM/NIGMS NIH HHS/United States
- DP2 OD006466/OD/NIH HHS/United States
- U54 GM072970/GM/NIGMS NIH HHS/United States
- R01-AI36929/AI/NIAID NIH HHS/United States
- R01 AI036929/AI/NIAID NIH HHS/United States
- R01 AI067712/AI/NIAID NIH HHS/United States
- T32 GM007276/GM/NIGMS NIH HHS/United States
- DP2OD006466/OD/NIH HHS/United States
- 1S10OD01227601/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

