Management of acute attacks of hereditary angioedema: role of ecallantide
- PMID: 25931832
- PMCID: PMC4404974
- DOI: 10.2147/JBM.S66825
Management of acute attacks of hereditary angioedema: role of ecallantide
Abstract
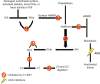
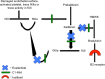
Hereditary angioedema (HAE) is characterized as an episodic swelling disorder with autosomal dominant inheritance. Clinical features include nonpitting edema of external or mucosal body surfaces, and patients often present with swelling of the extremities, abdominal pain, and swelling of the mouth and throat, which can lead to asphyxiation. Patients with HAE classically have no associated urticaria, which is often referred to as nonhistaminergic angioedema. Treatment for HAE involves long-term prophylaxis, short-term prophylaxis, and management of acute attacks. Up until the past few years, acute HAE episodes were predominately treated with supportive measures. Three classes of medications have recently been approved by the US Food and Drug Administration (FDA) for the management of acute HAE attacks. Ecallantide, a recombinant protein that acts as a reversible inhibitor of kallikrein, is currently indicated for acute attacks of HAE in those aged ≥12 years. In two randomized, double-blind, placebo-controlled, multicenter trials, EDEMA3 and EDEMA4, patients treated with 30 mg of ecallantide demonstrated statistically significant improvement in symptoms compared to those on placebo. In addition to its use as treatment for HAE, ecallantide has been used off label in the management of nonhistaminergic angioedema, not due to HAE. Ecallantide has shown promise in the treatment of these other forms; however, data are limited to mainly case reports at this time. Ecallantide is generally a safe and well-tolerated medication; however, based on reports of anaphylaxis, ecallantide does contain a black box warning. Due to the risk of anaphylaxis, ecallantide cannot be self-administered and must be given by a health care professional. Overall, ecallantide is a safe and effective medication for the treatment of acute attacks of HAE.
Keywords: 7- C1-Inhibitor; ACE-Inhibitor induced angioedema; Kallikrein; acquired angioedema; bradykinin; idiopathic angioedema; nonhistaminergic angioedema.
Figures
Similar articles
-
Ecallantide for the treatment of hereditary angiodema in adults.Clin Med Insights Cardiol. 2011;5:49-54. doi: 10.4137/CMC.S4434. Epub 2011 May 17. Clin Med Insights Cardiol. 2011. PMID: 21695090 Free PMC article.
-
Critical role of kallikrein in hereditary angioedema pathogenesis: a clinical trial of ecallantide, a novel kallikrein inhibitor.J Allergy Clin Immunol. 2007 Aug;120(2):416-22. doi: 10.1016/j.jaci.2007.04.028. Epub 2007 Jun 7. J Allergy Clin Immunol. 2007. PMID: 17559913 Clinical Trial.
-
Ecallantide for treatment of acute hereditary angioedema attacks: analysis of efficacy by patient characteristics.Allergy Asthma Proc. 2012 Mar-Apr;33(2):178-85. doi: 10.2500/aap.2012.33.3528. Allergy Asthma Proc. 2012. PMID: 22525395
-
Ecallantide: a plasma kallikrein inhibitor for the treatment of acute attacks of hereditary angioedema.Drugs Today (Barc). 2010 Aug;46(8):547-55. doi: 10.1358/dot.2010.46.8.1507205. Drugs Today (Barc). 2010. PMID: 20830315 Review.
-
Ecallantide for treatment of acute attacks of hereditary angioedema.Am J Health Syst Pharm. 2012 Apr 15;69(8):651-7. doi: 10.2146/ajhp110227. Am J Health Syst Pharm. 2012. PMID: 22472866 Review.
Cited by
-
Current and Prospective Targets of Pharmacologic Treatment of Hereditary Angioedema Types 1 and 2.Clin Rev Allergy Immunol. 2021 Aug;61(1):66-76. doi: 10.1007/s12016-021-08832-x. Epub 2021 Jan 9. Clin Rev Allergy Immunol. 2021. PMID: 33423210 Free PMC article. Review.
-
Self-Management Plans in Patients with Hereditary Angioedema: Strategies, Outcomes and Integration into Clinical Care.J Asthma Allergy. 2020 Apr 30;13:153-158. doi: 10.2147/JAA.S200900. eCollection 2020. J Asthma Allergy. 2020. PMID: 32431520 Free PMC article. Review.
-
The Interplay of COVID-19 and Hereditary Angioedema: Preventing an Acute Attack.Cureus. 2022 Sep 15;14(9):e29189. doi: 10.7759/cureus.29189. eCollection 2022 Sep. Cureus. 2022. PMID: 36507113 Free PMC article.
-
Prolonging the circulatory half-life of C1 esterase inhibitor via albumin fusion.PLoS One. 2024 Oct 23;19(10):e0305719. doi: 10.1371/journal.pone.0305719. eCollection 2024. PLoS One. 2024. PMID: 39441778 Free PMC article.
-
Component 1 Inhibitor Missense (Val480Met) Variant Is Associated With Gene Expression and Sepsis Development in Neonatal Lung Disease.Front Pediatr. 2022 May 20;10:779511. doi: 10.3389/fped.2022.779511. eCollection 2022. Front Pediatr. 2022. PMID: 35669402 Free PMC article.
References
-
- Berry A, Firszt R. Successful treatment of idiopathic angioedema with ecallantide. J Allergy Clin Immunol. 2013;1(3):297–298. - PubMed
-
- Zuraw BL. Hereditary angioedema. N Engl J Med. 2008;359(10):1027–1036. - PubMed
-
- Cicardi M, Agostoni A. Hereditary angioedema. N Engl J Med. 1996;334(25):1666–1667. - PubMed
-
- Bygum A. Hereditary angio-oedema in Denmark: a nationwide survey. Br J Dermatol. 2009;161(5):1153–1158. - PubMed
-
- Roche O, Blanch A, Caballero T, Sastre N, Callejo D, Lopez-Trascasa M. Hereditary angioedema due to C1 inhibitor deficiency: patient registry and approach to the prevalence in Spain. Ann Allergy Asthma Immunol. 2005;94(4):498–503. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

