Phase II multi-center clinical study on using S-1 to treat advanced breast cancer after resistance to anthracycline and taxane drugs in Chinese patients
- PMID: 25932284
- PMCID: PMC4402931
Phase II multi-center clinical study on using S-1 to treat advanced breast cancer after resistance to anthracycline and taxane drugs in Chinese patients
Abstract
Background: Treatment for metastatic breast cancer (MBC) in patients who have relapsed from anthracycline and taxane is difficult. S-1, an oral 5-FU derivative, has demonstrated a potential antitumor effect in patients with MBC. Thus, we evaluated the efficacy and safety of S-1 as second-line chemotherapy MBC patients in a phase II trial.
Methods: The study was conducted at seven centers in China and enrolled MBC patients who had previously relapsed from one chemotherapy regimen. The median progression-free survival (PFS) was the primary end point. The treatment schedule involved the administration of S-1 at a standard dose based on the body surface area (BSA) in 28-day cycles with consecutive administration followed by a 14-day rest, as follows: 40 mg twice daily if BSA < 1.25 m(2); 50 mg twice daily if 1.25 m(2) ≤ BSA ≥ 1.5 m(2); and 60 mg twice daily if BSA > 1.5 m(2).
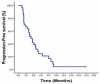
Results: Thirty-three patients were included in the analysis. S-1 demonstrated moderate efficacy with a PFS of 3.3 months, a response rate of 33.3%, and a disease control rate of 72.7%. The treatment was well-tolerated with mild-to-moderate toxicity. Grade 3 adverse events (AEs) occurred in 4 patients (2 with hyperbilirubinemia, 1 with anorexia, and 1 with vomiting). Grade 4 AEs were not observed.
Conclusion: S-1 demonstrated encouraging efficacy and safety in a prospective trial as second-line treatment in MBC patients. All AEs were manageable; however, bilirubin monitoring is recommended during treatment.
Keywords: Metastatic breast cancer; S-1; chemotherapy.
Figures
Similar articles
-
A Phase II Study of Irinotecan and Etoposide as Treatment for Refractory Metastatic Breast Cancer.Oncologist. 2019 Dec;24(12):1512-e1267. doi: 10.1634/theoncologist.2019-0516. Epub 2019 Aug 5. Oncologist. 2019. PMID: 31383812 Free PMC article. Clinical Trial.
-
A phase II study of biweekly S-1 and paclitaxel (SPA) as first-line chemotherapy in patients with metastatic or advanced gastric cancer.Cancer Chemother Pharmacol. 2015 Jul;76(1):197-203. doi: 10.1007/s00280-015-2782-z. Epub 2015 May 27. Cancer Chemother Pharmacol. 2015. PMID: 26013324 Clinical Trial.
-
An all-oral combination of metronomic cyclophosphamide plus capecitabine in patients with anthracycline- and taxane-pretreated metastatic breast cancer: a phase II study.Cancer Chemother Pharmacol. 2012 Feb;69(2):515-22. doi: 10.1007/s00280-011-1728-3. Epub 2011 Aug 27. Cancer Chemother Pharmacol. 2012. PMID: 21874317 Clinical Trial.
-
Pegylated liposomal doxorubicin (Lipo-Dox®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: an open-label, multi-center, non-comparative phase II study.BMC Cancer. 2015 May 21;15:423. doi: 10.1186/s12885-015-1433-4. BMC Cancer. 2015. PMID: 25994543 Free PMC article. Clinical Trial.
-
Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases.Clin Ther. 2008 Aug;30(8):1426-47. doi: 10.1016/j.clinthera.2008.08.008. Clin Ther. 2008. PMID: 18803986 Review.
Cited by
-
Multicentre, phase II study of eribulin in combination with S-1 in patients with advanced breast cancer.BMC Cancer. 2019 Oct 16;19(1):962. doi: 10.1186/s12885-019-6200-5. BMC Cancer. 2019. PMID: 31619197 Free PMC article. Clinical Trial.
References
-
- Blum JL, Barrios CH, Feldman N, Verma S, McKenna EF, Lee LF, Scotto N, Gralow J. Pooled analysis of individual patient data from capecitabine monotherapy clinical trials in locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2012;136:777–88. - PubMed
-
- Blum JL, Dieras V, Lo Russo PM, Horton J, Rutman O, Buzdar A, Osterwalder B. Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92:1759–68. - PubMed
-
- Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J. Clin. Oncol. 1999;17:485–93. - PubMed
-
- Scheithauer W, McKendrick J, Begbie S, Borner M, Burns WI, Burris HA, Cassidy J, Jodrell D, Koralewski P, Levine EL, Marschner N, Maroun J, Garcia-Alfonso P, Tujakowski J, Van Hazel G, Wong A, Zaluski J, Twelves C X-ACT Study Group. Oral capecitabine as an alternative to i. v. 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol. 2003;14:1735–43. - PubMed
-
- Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, McKendric J, Maroun J, Marshall J, Osterwalder B, Pérez-Manga G, Rosso R, Rougier P, Schilsky RL Capecitabine Colorectal Cancer Study Group. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002;13:566–75. - PubMed
LinkOut - more resources
Full Text Sources