Evaluation of effect of galvanic corrosion between nickel-chromium metal and titanium on ion release and cell toxicity
- PMID: 25932317
- PMCID: PMC4414949
- DOI: 10.4047/jap.2015.7.2.172
Evaluation of effect of galvanic corrosion between nickel-chromium metal and titanium on ion release and cell toxicity
Abstract
Purpose: The purpose of this study was to evaluate cell toxicity due to ion release caused by galvanic corrosion as a result of contact between base metal and titanium.
Materials and methods: It was hypothesized that Nickel (Ni)-Chromium (Cr) alloys with different compositions possess different corrosion resistances when contacted with titanium abutment, and therefore in this study, specimens (10×10×1.5 mm) were fabricated using commercial pure titanium and 3 different types of Ni-Cr alloys (T3, Tilite, Bella bond plus) commonly used for metal ceramic restorations. The specimens were divided into 6 groups according to the composition of Ni-Cr alloy and contact with titanium. The experimental groups were in direct contact with titanium and the control groups were not. After the samples were immersed in the culture medium - Dulbecco's modified Eagle's medium[DMEM] for 48 hours, the released metal ions were detected using inductively coupled plasma mass spectrometer (ICP-MS) and analyzed by the Kruskal-Wallis and Mann-Whitney test (P<.05). Mouse L-929 fibroblast cells were used for cell toxicity evaluation. The cell toxicity of specimens was measured by the 3-{4,5-dimethylthiazol-2yl}-2,5-diphenyltetrazolium bromide (MTT) test. Results of MTT assay were statistically analyzed by the two-way ANOVA test (P<.05). Post-hoc multiple comparisons were conducted using Tukey's tests.
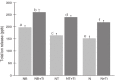
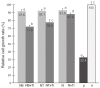
Results: The amount of metal ions released by galvanic corrosion due to contact between the base metal alloy and titanium was increased in all of the specimens. In the cytotoxicity test, the two-way ANOVA showed a significant effect of the alloy type and galvanic corrosion for cytotoxicity (P<.001). The relative cell growth rate (RGR) was decreased further on the groups in contact with titanium (P<.05).
Conclusion: The release of metal ions was increased by galvanic corrosion due to contact between base metal and titanium, and it can cause adverse effects on the tissue around the implant by inducing cytotoxicity.
Keywords: Cytotoxicity; Dental alloy; Galvanic corrosion; Ion release; Nickel-Chromium; Titanium abutment.
Figures
Similar articles
-
[Corrosion property and oxide film of dental casting alloys before and after porcelain firing].Zhonghua Kou Qiang Yi Xue Za Zhi. 2011 Mar;46(3):172-6. Zhonghua Kou Qiang Yi Xue Za Zhi. 2011. PMID: 21575441 Chinese.
-
Diamondlike carbon coating as a galvanic corrosion barrier between dental implant abutments and nickel-chromium superstructures.Int J Oral Maxillofac Implants. 2013 Jul-Aug;28(4):1037-47. doi: 10.11607/jomi.3091. Int J Oral Maxillofac Implants. 2013. PMID: 23869362
-
Corrosive and cytotoxic properties of compact specimens and microparticles of Ni-Cr dental alloy.J Prosthodont. 2014 Apr;23(3):221-6. doi: 10.1111/jopr.12100. Epub 2013 Oct 7. J Prosthodont. 2014. PMID: 24118161
-
Fracture of dental implants: literature review and report of a case.Implant Dent. 2002;11(2):137-43. doi: 10.1097/00008505-200204000-00014. Implant Dent. 2002. PMID: 12078595 Review.
-
Electrogalvanism in Oral Implantology: A Systematic Review.Int J Dent. 2022 Aug 5;2022:4575416. doi: 10.1155/2022/4575416. eCollection 2022. Int J Dent. 2022. PMID: 36034476 Free PMC article. Review.
Cited by
-
Preparation of capsaicin-loaded ultrafine fiber film and its application in the treatment of oral ulcers in rats.Sci Rep. 2023 Aug 25;13(1):13941. doi: 10.1038/s41598-023-40375-3. Sci Rep. 2023. PMID: 37626141 Free PMC article.
-
Corrosive Studies of a Prosthetic Ni-Cr Alloy Coated with Ti(C,N) Type Layers.Materials (Basel). 2022 Mar 27;15(7):2471. doi: 10.3390/ma15072471. Materials (Basel). 2022. PMID: 35407804 Free PMC article.
-
Comparing Castability of Nickel-Chromium, Cobalt-Chromium, and Non-Precious Gold Color Alloys, Using two Different Casting Techniques.J Dent (Shiraz). 2022 Mar;23(1):7-12. doi: 10.30476/DENTJODS.2021.87573.1275. J Dent (Shiraz). 2022. PMID: 35291681 Free PMC article.
-
Massive osteolysis due to galvanic corrosion after total knee arthroplasty: a rare cause for early revision?J Surg Case Rep. 2020 Feb 7;2020(2):rjaa002. doi: 10.1093/jscr/rjaa002. eCollection 2020 Feb. J Surg Case Rep. 2020. PMID: 32047588 Free PMC article.
-
Effect of SiC Abrasive Blasting Parameters on the Quality of the Ceramic and Ni-Cr Dental Alloy Joint.Materials (Basel). 2022 Jan 26;15(3):964. doi: 10.3390/ma15030964. Materials (Basel). 2022. PMID: 35160910 Free PMC article.
References
-
- Wataha JC. Alloys for prosthodontic restorations. J Prosthet Dent. 2002;87:351–363. - PubMed
-
- Wataha JC, Messer RL. Casting alloys. Dent Clin North Am. 2004;48:vii–viii. 499–512. - PubMed
-
- Corso PP, Jr, German RM, Simmons HD., Jr Corrosion evaluation of gold-based dental alloys. J Dent Res. 1985;64:854–859. - PubMed
-
- Tuna SH, Pekmez NO, Keyf F, Canli F. The influence of the pure metal components of four different casting alloys on the electrochemical properties of the alloys. Dent Mater. 2009;25:1096–1103. - PubMed
-
- Lappalainen R, Yli-Urpo A. Release of elements from some gold alloys and amalgams in corrosion. Scand J Dent Res. 1987;95:364–368. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

