GMP-compliant (68)Ga radiolabelling in a conventional small-scale radiopharmacy: a feasible approach for routine clinical use
- PMID: 25932354
- PMCID: PMC4412871
- DOI: 10.1186/s13550-015-0105-3
GMP-compliant (68)Ga radiolabelling in a conventional small-scale radiopharmacy: a feasible approach for routine clinical use
Abstract
Background: The number of routine care patient examinations with (68)Ga radiopharmaceuticals is still relatively limited, probably caused by the presumed need for large investments in hot cells, automated synthesis modules, laboratory equipment and validation efforts. Our aim was to set up the preparation of (68)Ga-DOTA-NOC in compliance with all current European Union-Good Manufacturing Practices (EU-GMP), current Good Radiopharmacy Practice (cGRPP) and European Pharmacopoeia (Ph. Eur.) guidance but without the availability of a hot cell and gas chromatography (GC), high-performance liquid chromatography (HPLC) and atomic absorption spectrometry (AAS) equipment.
Methods: A risk-based approach was applied to align preparation conditions with applicable regulations, together with a validation of a thin-layer chromatography (ITLC) method to replace HPLC as modality for examining radiochemical purity.
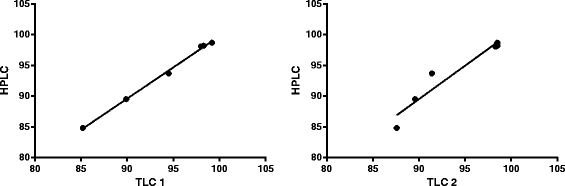
Results: Using an internally shielded labelling module for manual operation, a (68)Ga-DOTA-NOC labelling procedure was set up that meets all applicable Ph. Eur. specifications. The applied ITLC method showed very good correlation with HPLC results (r = 0.961) and was able to detect relevant deviations in radiolabelling procedures. All identified quality assurance aspects were made compliant with EU-GMP and cGRPP guidance.
Conclusions: We consider the described configuration and validation approach feasible for many conventional small-scale radiopharmacies, something that could help to increase the availability of (68)Ga radiopharmaceuticals to a large number of patients.
Keywords: 68Ga PET imaging; DOTA-NOC; GMP; NET; Octreotide; Validation.
Figures
Similar articles
-
Validation of Quality Control Parameters of Cassette-Based Gallium-68-DOTA-Tyr3-Octreotate Synthesis.Indian J Nucl Med. 2020 Oct-Dec;35(4):291-298. doi: 10.4103/ijnm.IJNM_66_20. Epub 2020 Oct 21. Indian J Nucl Med. 2020. PMID: 33642752 Free PMC article.
-
A Specific HPLC Method to Determine Residual HEPES in [68Ga]Ga-Radiopharmaceuticals: Development and Validation.Molecules. 2022 Jul 13;27(14):4477. doi: 10.3390/molecules27144477. Molecules. 2022. PMID: 35889351 Free PMC article.
-
Guideline on current good radiopharmacy practice (cGRPP) for the small-scale preparation of radiopharmaceuticals.EJNMMI Radiopharm Chem. 2021 Feb 12;6(1):8. doi: 10.1186/s41181-021-00123-2. EJNMMI Radiopharm Chem. 2021. PMID: 33580358 Free PMC article.
-
Automated synthesis and quality control of [68Ga]Ga-PentixaFor using the Gaia/Luna Elysia-Raytest module for CXCR4 PET imaging.EJNMMI Radiopharm Chem. 2023 Feb 7;8(1):4. doi: 10.1186/s41181-023-00187-2. EJNMMI Radiopharm Chem. 2023. PMID: 36749409 Free PMC article.
-
Overview and perspectives on automation strategies in (68)Ga radiopharmaceutical preparations.Recent Results Cancer Res. 2013;194:17-31. doi: 10.1007/978-3-642-27994-2_2. Recent Results Cancer Res. 2013. PMID: 22918752 Review.
Cited by
-
Comparison of Radiochemical and Chemical Impurities in Liquid Wastes of Two Different 68Ge/68Ga Generators used in Nuclear Medicine PET Chemistry.Mol Imaging Radionucl Ther. 2021 Feb 9;30(1):34-38. doi: 10.4274/mirt.galenos.2020.58569. Mol Imaging Radionucl Ther. 2021. PMID: 33586405 Free PMC article.
-
Good practices for 68Ga radiopharmaceutical production.EJNMMI Radiopharm Chem. 2022 Oct 22;7(1):27. doi: 10.1186/s41181-022-00180-1. EJNMMI Radiopharm Chem. 2022. PMID: 36271969 Free PMC article. Review.
-
Convenient Preparation of [(68)Ga]DKFZ-PSMA-11 Using a Robust Single-Vial Kit and Demonstration of Its Clinical Efficacy.Mol Imaging Biol. 2016 Jun;18(3):420-7. doi: 10.1007/s11307-016-0943-z. Mol Imaging Biol. 2016. PMID: 26983703 Clinical Trial.
-
On the automated radiosynthesis of pharmaceutical grade [68Ga]Ga-Pentixafor, its pre-clinical evaluation, clinical application and radiation dosimetry aspects.Sci Rep. 2025 Feb 22;15(1):6476. doi: 10.1038/s41598-024-84096-7. Sci Rep. 2025. PMID: 39987209 Free PMC article.
-
Automated preparation of clinical grade [68Ga]Ga-DOTA-CP04, a cholecystokinin-2 receptor agonist, using iPHASE MultiSyn synthesis platform.EJNMMI Radiopharm Chem. 2019 Aug 23;4(1):23. doi: 10.1186/s41181-019-0067-2. EJNMMI Radiopharm Chem. 2019. PMID: 31659509 Free PMC article.
References
-
- Mäcke HR, Smith-Jones P, Maina T, Stolz B, Albert R, Bruns C, et al. New octreotide derivatives for in vivo targeting of somatostatin receptor-positive tumors for single photon emission computed tomography (SPECT) and positron emission tomography (PET) Horm Metab Res Suppl. 1993;27:12–7. - PubMed
-
- Hofmann M, Mäcke HR, Börner R. Weckesser E, Schöffski P, Oei ML, Schumacher J, Henze M, Heppeler A, Meyer GJ, Knapp WH. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001;28:1751–7 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous