The mechanisms of action of vaccines containing aluminum adjuvants: an in vitro vs in vivo paradigm
- PMID: 25932368
- PMCID: PMC4406982
- DOI: 10.1186/s40064-015-0972-0
The mechanisms of action of vaccines containing aluminum adjuvants: an in vitro vs in vivo paradigm
Abstract
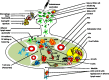
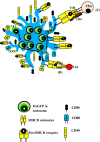
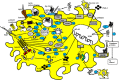
Adjuvants such as the aluminum compounds (alum) have been dominantly used in many vaccines due to their immunopotentiation and safety records since 1920s. However, how these mineral agents influence the immune response to vaccination remains elusive. Many hypotheses exist as to the mode of action of these adjuvants, such as depot formation, antigen (Ag) targeting, and the induction of inflammation. These hypotheses are based on many in vitro and few in vivo studies. Understanding how cells interact with adjuvants in vivo will be crucial to fully understanding the mechanisms of action of these adjuvants. Interestingly, how alum influences the target cell at both the cellular and molecular level, and the consequent innate and adaptive responses, will be critical in the rational design of effective vaccines against many diseases. Thus, in this review, mechanisms of action of alum have been discussed based on available in vitro vs in vivo evidences to date.
Keywords: Alum internalization; Antigen targeting; Depot; Inflammasome; Innate and adaptive.
Figures
Similar articles
-
Mechanisms of action of adjuvants.Front Immunol. 2013 May 16;4:114. doi: 10.3389/fimmu.2013.00114. eCollection 2013. Front Immunol. 2013. PMID: 23720661 Free PMC article.
-
Microcrystalline Tyrosine and Aluminum as Adjuvants in Allergen-Specific Immunotherapy Protect from IgE-Mediated Reactivity in Mouse Models and Act Independently of Inflammasome and TLR Signaling.J Immunol. 2018 May 1;200(9):3151-3159. doi: 10.4049/jimmunol.1800035. Epub 2018 Mar 28. J Immunol. 2018. PMID: 29592962 Free PMC article.
-
Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation.Expert Rev Vaccines. 2007 Oct;6(5):685-98. doi: 10.1586/14760584.6.5.685. Expert Rev Vaccines. 2007. PMID: 17931150 Review.
-
Novel identified aluminum hydroxide-induced pathways prove monocyte activation and pro-inflammatory preparedness.J Proteomics. 2018 Mar 20;175:144-155. doi: 10.1016/j.jprot.2017.12.021. Epub 2018 Jan 6. J Proteomics. 2018. PMID: 29317357
-
Vaccine Adjuvants: from 1920 to 2015 and Beyond.Vaccines (Basel). 2015 Apr 16;3(2):320-43. doi: 10.3390/vaccines3020320. Vaccines (Basel). 2015. PMID: 26343190 Free PMC article. Review.
Cited by
-
Adult multisystem inflammatory syndrome in a patient who recovered from COVID-19 postvaccination.BMJ Case Rep. 2021 Apr 21;14(4):e242060. doi: 10.1136/bcr-2021-242060. BMJ Case Rep. 2021. PMID: 33883119 Free PMC article.
-
Sequestering of damage-associated molecular patterns (DAMPs): a possible mechanism affecting the immune-stimulating properties of aluminium adjuvants.Immunol Res. 2017 Dec;65(6):1164-1175. doi: 10.1007/s12026-017-8972-5. Immunol Res. 2017. PMID: 29181774 Free PMC article.
-
BECC438b TLR4 agonist supports unique immune response profiles from nasal and muscular DTaP pertussis vaccines in murine challenge models.Infect Immun. 2024 Mar 12;92(3):e0022323. doi: 10.1128/iai.00223-23. Epub 2024 Feb 7. Infect Immun. 2024. PMID: 38323817 Free PMC article.
-
Thermosensitive PLGA-PEG-PLGA Hydrogel as Depot Matrix for Allergen-Specific Immunotherapy.Pharmaceutics. 2022 Jul 22;14(8):1527. doi: 10.3390/pharmaceutics14081527. Pharmaceutics. 2022. PMID: 35893787 Free PMC article.
-
The immunological and pharmacokinetic evaluation of Lipid-PLGA hybrid nanoparticle-based oxycodone vaccines.Biomaterials. 2025 Feb;313:122758. doi: 10.1016/j.biomaterials.2024.122758. Epub 2024 Aug 18. Biomaterials. 2025. PMID: 39182328
References
-
- Anonymous . United States Minimum Requirements. Tetanus and Diphtheria Toxoids Combined Precipitated, Adsorbed (For Adult Use) Bethesda: US Department of Health, Education and Welfare, National Institute of Health; 1956.
-
- Authier FJ, Sauvat S, Christov C, Chariot P, Raisbeck G, Poron MF, Yiou F, Gherardi R (2006) AlOH3-adjuvanted vaccine-induced macrophagic myofasciitis in rats is influenced by the genetic background. Neuromuscul Disord 16(5):347–352. doi:S0960-8966(06)00036-8 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

