Phase ii, randomized, placebo-controlled, 90-day study of emixustat hydrochloride in geographic atrophy associated with dry age-related macular degeneration
- PMID: 25932553
- PMCID: PMC4452434
- DOI: 10.1097/IAE.0000000000000606
Phase ii, randomized, placebo-controlled, 90-day study of emixustat hydrochloride in geographic atrophy associated with dry age-related macular degeneration
Abstract
Purpose: This study assessed the safety, tolerability, and pharmacodynamics of emixustat hydrochloride (ACU-4429), a novel visual cycle modulator, in subjects with geographic atrophy associated with dry age-related macular degeneration.
Methods: Subjects were randomly assigned to oral emixustat (2, 5, 7, or 10 mg once daily) or placebo (3:1 ratio) for 90 days. Recovery of rod photoreceptor sensitivity after a photobleach was measured by electroretinography. Safety evaluations included analysis of adverse events and ophthalmic examinations.
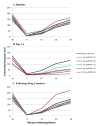
Results: Seventy-two subjects (54 emixustat and 18 placebo) were evaluated. Emixustat suppressed rod photoreceptor sensitivity in a dose-dependent manner. Suppression plateaued by Day 14 and was reversible within 7 days to 14 days after drug cessation. Most systemic adverse events were not considered treatment related. Dose-related ocular adverse events (chromatopsia, 57% emixustat vs. 17% placebo and delayed dark adaptation, 48% emixustat vs. 6% placebo) were mild to moderate in severity, and the majority resolved on study or within 7 days to 14 days after study drug cessation. Reversibility of these adverse events with long-term administration, however, is undetermined.
Conclusion: In this Phase II study, emixustat produced a dose-dependent reversible effect on rod function that is consistent with the proposed mechanism of action. These results support further testing of emixustat for the treatment of geographic atrophy associated with dry age-related macular degeneration.
Conflict of interest statement
Figures

References
-
- de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–85. - PubMed
-
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–17. - PubMed
-
- Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. - PubMed
-
- Pascolini D, Mariotti SPM. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

