The lidocaine metabolite N-ethylglycine has antinociceptive effects in experimental inflammatory and neuropathic pain
- PMID: 25932687
- PMCID: PMC4617288
- DOI: 10.1097/j.pain.0000000000000206
The lidocaine metabolite N-ethylglycine has antinociceptive effects in experimental inflammatory and neuropathic pain
Abstract
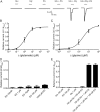
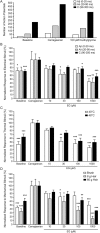
Glycine transporter 1 (GlyT1) plays a crucial role in regulating extracellular glycine concentrations and might thereby constitute a new drug target for the modulation of glycinergic inhibition in pain signaling. Consistent with this view, inhibition of GlyT1 has been found to induce antinociceptive effects in various animal pain models. We have shown previously that the lidocaine metabolite N-ethylglycine (EG) reduces GlyT1-dependent glycine uptake by functioning as an artificial substrate for this transporter. Here, we show that EG is specific for GlyT1 and that in rodent models of inflammatory and neuropathic pain, systemic treatment with EG results in an efficient amelioration of hyperalgesia and allodynia without affecting acute pain. There was no effect on motor coordination or the development of inflammatory edema. No adverse neurological effects were observed after repeated high-dose application of EG. EG concentrations both in blood and spinal fluid correlated with an increase of glycine concentration in spinal fluid. The time courses of the EG and glycine concentrations corresponded well with the antinociceptive effect. Additionally, we found that EG reduced the increase in neuronal firing of wide-dynamic-range neurons caused by inflammatory pain induction. These findings suggest that systemically applied lidocaine exerts antihyperalgesic effects through its metabolite EG in vivo, by enhancing spinal inhibition of pain processing through GlyT1 modulation and subsequent increase of glycine concentrations at glycinergic inhibitory synapses. EG and other substrates of GlyT1, therefore, may be a useful therapeutic agent in chronic pain states involving spinal disinhibition.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures






References
-
- Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. PGE(2) selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci 2002;5:34–40. - PubMed
-
- Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, Nurmikko T; European Federation of Neurological S. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 2010;17:1113–e88. - PubMed
-
- Backonja MM. Neuropathic pain therapy: from bench to bedside. Semin Neurol 2012;32:264–8. - PubMed
-
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19. - PubMed
-
- Barthel F, Urban A, Schlosser L, Eulenburg V, Werdehausen R, Brandenburger T, Aragon C, Bauer I, Hermanns H. Long-term application of glycine transporter inhibitors acts antineuropathic and modulates spinal N-methyl-D-aspartate receptor subunit NR-1 expression in rats. Anesthesiology 2014;121:160–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

