Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure
- PMID: 25932909
- PMCID: PMC4949714
- DOI: 10.1111/nph.13435
Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure
Abstract
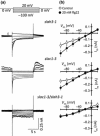
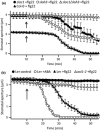
During infection plants recognize microbe-associated molecular patterns (MAMPs), and this leads to stomatal closure. This study analyzes the molecular mechanisms underlying this MAMP response and its interrelation with ABA signaling. Stomata in intact Arabidopsis thaliana plants were stimulated with the bacterial MAMP flg22, or the stress hormone ABA, by using the noninvasive nanoinfusion technique. Intracellular double-barreled microelectrodes were applied to measure the activity of plasma membrane ion channels. Flg22 induced rapid stomatal closure and stimulated the SLAC1 and SLAH3 anion channels in guard cells. Loss of both channels resulted in cells that lacked flg22-induced anion channel activity and stomata that did not close in response to flg22 or ABA. Rapid flg22-dependent stomatal closure was impaired in plants that were flagellin receptor (FLS2)-deficient, as well as in the ost1-2 (Open Stomata 1) mutant, which lacks a key ABA-signaling protein kinase. By contrast, stomata of the ABA protein phosphatase mutant abi1-1 (ABscisic acid Insensitive 1) remained flg22-responsive. These data suggest that the initial steps in flg22 and ABA signaling are different, but that the pathways merge at the level of OST1 and lead to activation of SLAC1 and SLAH3 anion channels.
Keywords: ABA; Arabidopsis thaliana; S-type anion channel; flg22; guard cells; innate immunity; microbe-associated molecular pattern (MAMP); stomata.
© 2015 The Authors. New Phytologist © 2015 New Phytologist Trust.
Figures





References
-
- Amatore C, Arbault S, Bouton C, Coffi K, Drapier JC, Ghandour H, Tong YH. 2006. Monitoring in real time with a microelectrode the release of reactive oxygen and nitrogen species by a single macrophage stimulated by its membrane mechanical depolarization. ChemBioChem 7: 653–661. - PubMed
-
- Bauer Z, Gomez‐Gomez L, Boller T, Felix G. 2001. Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor‐binding sites. Journal of Biological Chemistry 276: 45669–45676. - PubMed
-
- Boller T, Felix G. 2009. A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annual Review of Plant Biology 60: 379–406. - PubMed
-
- Boudsocq M, Droillard MJ, Regad L, Lauriere C. 2012. Characterization of Arabidopsis calcium‐dependent protein kinases: activated or not by calcium? Biochemical Journal 447: 291–299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

