Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy
- PMID: 25932963
- PMCID: PMC4830087
- DOI: 10.1038/modpathol.2015.53
Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy
Abstract
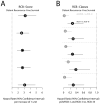
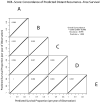
The residual cancer burden index was developed as a method to quantify residual disease ranging from pathological complete response to extensive residual disease. The aim of this study was to evaluate the inter-Pathologist reproducibility in the residual cancer burden index score and category, and in their long-term prognostic utility. Pathology slides and pathology reports of 100 cases from patients treated in a randomized neoadjuvant trial were reviewed independently by five pathologists. The size of tumor bed, average percent overall tumor cellularity, average percent of the in situ cancer within the tumor bed, size of largest axillary metastasis, and number of involved nodes were assessed separately by each pathologist and residual cancer burden categories were assigned to each case following calculation of the numerical residual cancer burden index score. Inter-Pathologist agreement in the assessment of the continuous residual cancer burden score and its components and agreement in the residual cancer burden category assignments were analyzed. The overall concordance correlation coefficient for the agreement in residual cancer burden score among pathologists was 0.931 (95% confidence interval (CI) 0.908-0.949). Overall accuracy of the residual cancer burden score determination was 0.989. The kappa coefficient for overall agreement in the residual cancer burden category assignments was 0.583 (95% CI 0.539-0.626). The metastatic component of the residual cancer burden index showed stronger concordance between pathologists (overall concordance correlation coefficient=0.980; 95% CI 0.954-0.992), than the primary component (overall concordance correlation coefficient=0.795; 95% CI 0.716-0.853). At a median follow-up of 12 years residual cancer burden determined by each of the pathologists had the same prognostic accuracy for distant recurrence-free and survival (overall concordance correlation coefficient=0.995; 95% CI 0.989-0.998). Residual cancer burden assessment is highly reproducible, with reproducible long-term prognostic significance.
Figures

References
-
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. - PubMed
-
- Kuerer HM, Neuman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. - PubMed
-
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTneoBC pooled analysis. The Lancet. 2014;384:164–172. - PubMed
-
- Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

