Efavirenz-Based Regimens in Antiretroviral-Naive HIV-Infected Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- PMID: 25933004
- PMCID: PMC4416921
- DOI: 10.1371/journal.pone.0124279
Efavirenz-Based Regimens in Antiretroviral-Naive HIV-Infected Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
Efavirenz, a non-nucleoside reverse-transcriptase inhibitor (NNRTI) is one of the most commonly prescribed antiretroviral drugs. The present article provides a systematic overview and meta-analysis of clinical trials comparing efavirenz and other active drugs currently recommended for treatment of HIV-infected, antiretroviral-naive patients. Electronic databases (Pubmed, Embase, the Cochrane Library, Trip Database) were searched up till 23 December 2013 for randomized controlled clinical trials published as a peer-reviewed papers, and concerning efavirenz-based regimens used as initial treatment for HIV infection. Thirty-four studies were included in the systematic review, while twenty-six trials were suitable for the meta-analysis. Efavirenz was compared with drugs from four different classes: NNRTIs other than efavirenz (nevirapine or rilpivirine), integrase strand transfer inhibitors (InSTIs), ritonavir-boosted protease inhibitors (bPI) and chemokine (C-C motif) receptor 5 (CCR5) antagonists (maraviroc), all of them were added to the background regimen. Results of the current meta-analysis showed that efavirenz-based regimens were equally effective as other recommended regimens based on NNRTI, ritonavir-boosted PI or CCR5 antagonist in terms of efficacy outcomes (disease progression and/or death, plasma viral HIV RNA <50 copies/ml) while statistically significant more patients treated with InSTI achieved plasma viral load <50 copies/ml at week 48. In comparison with both InSTI-based and CCR5-based therapy, efavirenz-based treatment was associated with a higher risk of therapy discontinuation due to adverse events. However, comparisons of efevirenz-based treatment with InSTI-based and CCR5-based therapy were based on a limited number of trials, therefore, conclusions from these two comparisons must be confirmed in further reliable randomized controlled studies. Results of our meta-analysis support the present clinical guidelines for antiretroviral-naive, HIV-infected patients, in which efavirenz is one of the most preferred regimens in the analyzed population. Beneficial safety profile of InSTI-based and CCR5-based therapy over efavirenz-based treatment needs further studies.
Conflict of interest statement
Figures
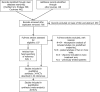


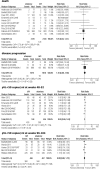

References
-
- Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, Flaniqan TP, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135: 17–26. - PubMed
-
- Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet 1998;352: 1725–1730. - PubMed
-
- Gulick RM, Ribaudo HJ, Shikuma CM, Lustgarten S, Squires KE, Meyer WA 3rd. AIDS Clinical Trials Group Study A5095 Team. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350(18): 1850–1861. - PubMed
-
- Gallant J, DeJesus E, Arribas J, Pozniak A, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354: 251–260. - PubMed
-
- Vrouenraets SM, Wit FW, van Tongeren J, Lange JM. Efavirenz: a review. Expert Opin Pharmacother. 2007;8(6): 851–871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

